Measuring the concentration of aqueous solutions containing one component can be easy. There are numerous techniques that accomplish this goal, such as density measurement or titration. Aqueous solutions that contain two dissolved components, however, present entirely different challenges. With two compounds in solution, one compound can easily influence the measurement of the other, leading to errors and inaccuracies. It is far more difficult to accurately determine the concentration of two materials when they are both in the same solution.

Anton Paar DSA 5000 M Density and Sound Velocity Meter
In order to overcome this difficulty, an analytical technique is required that can separate the two components based on some chemical or physical property that is different between the two components. For instance, gas chromatography will use a stationary phase to separate the components based on different traveling times before they reach the detector for analysis. This technique is very useful, but it can also be expensive in terms of equipment costs, training, labor and materials. Certain compounds can be titrated individually without influencing other materials in the solution. However, this requires twice the work to titrate both compounds, and not all compounds can be titrated individually. Additionally, titrations often involve hazardous chemicals, ongoing expenditures for solvents and reagents, and can be time consuming.
Density measurement can provide a very accurate concentration determination for a binary solution if you have a concentration table for your compound. However, the presence of a second material in solution will cause changes to the density reading that will influence the concentration measurement. It is possible to add a second physical property to your density measurement in order to take into account the presence of a second compound. Sound velocity is easily measured in liquids and is physically independent of density. A density and sound velocity analyzer is capable of measuring both quantities simultaneously and can use the combined data to determine the concentration of two unique components in an aqueous solution.
Certain common ternary solutions have already been well researched and have established density and sound velocity data. One of the most common applications is sugar inversion, where the conversion of sucrose into fructose and glucose can be monitored by means of density and sound velocity. Other examples include applications during chemical production processes. For instance, density and sound velocity measurement can be used for quality control during the production of formalin, a solution of methanol, formaldehyde and water, or with solutions of sulfuric acid, oleum and water.
A Short How-to:
Determine Your Concentration Range for Both Components
Depending on the application, the concentration ranges for both compounds should be defined to fit both the desired application and desired accuracy. A wider measuring range might lead to a decreased accuracy, so it may be necessary to define multiple, smaller concentration ranges. Most modern density and sound velocity analyzers are able to accommodate equations for multiple ranges, allowing flexibility and increased accuracy.
Additionally, samples can also be measured at various temperatures to include a temperature range in addition to a concentration range. However, since density and sound velocity analyzers can equilibrate the sample to a chosen temperature, this additional parameter can be used as an option.
Prepare Your Reference Samples
In order to create a sample specific concentration formula, well-defined reference samples that vary in the concentration of both components within the desired measuring range need to be prepared for initial measurements to determine the relationship between density, sound velocity, and concentration. You can choose the step graduation between sample concentrations based on individual need. A smaller step will likely give greater accuracy but also mean more samples to prepare and measure, while a wider step will have fewer samples but may be less accurate.
It is important to use pure compounds to make these reference samples in order to avoid impurities and any resultant inaccuracies in the measurement.
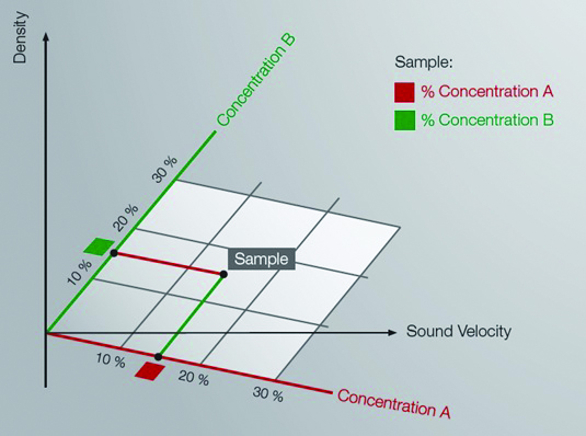
Concentration of two components in a ternary solution can be determined by the measurement of density and sound velocity.
Measure Your Reference Samples
This is the most time-consuming step. The reference samples must be measured to determine their density and sound velocity values. The subsequent concentration determination is only as good as the reference measurements. Achieving the highest precision in your sample handling procedure ensures the highest accuracy of the concentration formula. The raw data is then used to generate the mathematical coefficients to make an equation to calculate the concentration of your sample components within the defined measuring range.
Calculate the Coefficients
The manufacturer of the density and sound velocity meter will work with you to calculate the coefficients for the concentration formula. The final result will be a set of coefficients that will accurately define the concentration of your compounds within the desired measuring range. These coefficients can then be set up as a user formula in your density and sound velocity meter, allowing you to measure your sample and automatically obtain the concentration results of both components at the same time.
Density and sound velocity analysis is strongly application dependent, and depends entirely upon the density and sound velocity values of the sample reacting differently to changes in the concentration of the two components. However, because of the relative ease of the measurement, it is worth investigating to see if this measurement technique is a viable option.
