Total organic carbon (TOC) is a rapid method that analyzes for organic carbon and expresses the result as the amount of carbon found. It is a nonspecific method unable to distinguish between various organic species and indicates only that organic carbon compounds are present. TOC analyzers operate by determining the amount of total carbon in a sample aliquot. Total carbon consists of inorganic and organic carbon. Inorganic carbon, present as carbonate or bicarbonate ions, must be removed or quantified prior to the analysis of organic carbon. Once the inorganic carbon is removed, subsequent analysis of the sample aliquot assumes that all carbon remaining is organic.
Discussion
Methodology used to remove inorganic carbon relies on acidification that converts all bicarbonate and carbonate ions to carbon dioxide that is purged out of the sample using an inert gas. If quantification of inorganic carbon is desired, it is purged into a detector; otherwise it is vented to atmosphere. Once inorganic carbon is removed, the remaining organic carbon is oxidized to carbon dioxide that is purged by the inert gas into the detector.
Carbon measurement techniques
In the 1630s, Flemish scientist Jan Baptist van Helmont identified the gas emitted by the burning of wood as carbon dioxide. In 1756, Joseph Black demonstrated that carbon dioxide occurred in natural air and could be created from other compounds. While doing research on magnesium carbonates, Black invented the analytical balance and used it to measure carbon dioxide by loss on ignition (LOI). The LOI test, in which samples are heated and reduction in mass is measured, is the first quantitative test for carbon.
Organic matter in soil has traditionally been measured by LOI or chemical oxidation using dichromate solution. The dichromate, present as hexavalent chromium, reacts with reducing organic carbon in strong acid solution to form trivalent chromium. Titration of the unused hexavalent chromium with ferrous iron yields a method capable of estimating the organic carbon present in a sample.
A steel or coal sample can be placed in a furnace or heated tube and, in the presence of oxygen, the carbon converts to carbon dioxide. The carbon dioxide can be collected and measured or it can be determined by a carbon dioxide-specific detector. The initial steel analysis apparatus provides a basis for the modern TOC analyzer. In 1924, T.D. Yensen of the Westinghouse Electric and Manufacturing Company patented a “measuring device” that placed steel samples in a horizontal 1000 ºC furnace that combusted carbon in an oxygen carrier gas and collected the

Figure 1 – Ampules similar to those used by Menzel and Vacarro.
CO2cryogenically. In 1948, American Cyanamid patented an infrared (IR) gas analyzer, and in 1967 James Teal at Dow Chemical Company patented a “Method and Apparatus for Determination of Total Carbon Content in Aqueous Systems.” This apparatus is a combustion system that is similar to Yensen’s device and manually injects aqueous samples directly, using a syringe, into a stream of oxygen flowing through a 700–900 ºC furnace measuring the CO2 generated with infrared absorbance. The patent states that previously accepted methods for the determination of carbon in water were based on chemical oxidation methods at moderate temperatures. Teal’s device appears to be the first combustion TOC analyzer for water, and the previous method he is referring to is likely the chemical oxygen demand (COD) test. The method reported an analytical range of 2–500 ppm carbon and 98% or better combustion efficiency of all organic compounds tested.
Frustrated with an inability to achieve lower levels of detection on seawater using existing TOC combustion analyzers (recall that Teal’s analyzer has a lower limit of 2 mg/L), Menzel and Vaccarro1 devised an ampule-based wet chemical oxidation technique based on earlier work by R.F. Wilson.2 Wilson digested seawater samples using sodium persulfate at 100 ºC. Menzel and Vacarro’s ampule method allowed the simultaneous processing of large numbers of samples. Figure 1 shows ampules similar to those used by Menzel and Vacarro.
In 1965, Alan Fredericks and Donald W. Hood developed a TOC method based on Menzel and Vacarro’s ampule method that determined TOC in seawater using gas chromatography. This gas chromatographic method was later adapted to use an IR detector, and a newly formed company, Oceanographic Institute Corporation (OIC), commercialized the instrument. This new TOC analyzer digested samples using persulfate chemical oxidation by autoclaving samples enclosed in ampules. An autosampler broke the ampule and swept the CO2 gas into an IR detector. This instrument was capable of analyzing carbon in seawater to as low as 0.2 mg/L. The ampules had a significant advantage in that samples could be collected and sealed at sea pending subsequent digestion and analysis on land.
Ehrhard3 developed a TOC method using a Technicon AutoAnalyzer. This method combined continuous flow, ultraviolet irradiation and persulfate oxidation and collected the CO2 generated into a dilute sodium hydroxide solution measuring carbon by a change in conductivity. Cauwet4 improved on Ehrhard’s original procedure by optimizing pH, persulfate concentration, UV and IR detection.

Figure 2 – Shimadzu TOC-L HTCO TOC analyzer.
In 1988, Sugimara and Suzuki5 reported on a high-temperature catalytic oxidation (HTCO) method for the analysis of seawater by direct injection of 200 μL of sample into a 680 ºC furnace containing a platinum catalyst of the TOC-L HTCO TOC analyzer (Shimadzu Scientific Instruments, Columbia, Md.) (seeFigure 2). The method was rapid and precise and allowed shipboard
analysis. Moreover, it demonstrated higher TOC levels in seawater than previous methods, namely Menzel and Vacarro’s, spurring a debate on whether there is undetected carbon by chemical oxidation, or whether the HTCO method produces erroneously high results. After much research it was determined that both arguments were valid. Initial results generated by HTCO did not properly compensate for high blanks caused by carbon buildup within the combustion tube; however, even when compensating for blank values, the HTCO results were still slightly higher. Humic acids, bacteria, vegetation and certain high-molecular-weight molecules are oxidized with greater efficiency using HTCO. The higher oxidation efficiency of HTCO loses its value at lower concentrations (below about 1.0 mg/L of carbon) since HTCO is limited in sample volume compared to chemical oxidation (Standard Methods for the Examination of Water and Wastewater Method 5310).
Conclusion
TOC analysis attempts to measure carbon contained in organic molecules and report results as a single value. The value obtained is dependent on the oxidation technique and no single oxidation technique is adequate for every purpose. While HTCO has higher oxidation efficiency, the smaller sample volumes introduce sampling error at concentrations below about 1.0 mg/L of carbon; the lower oxidation efficiency of chemical methods is offset by the ability to digest fairly large sample volumes.
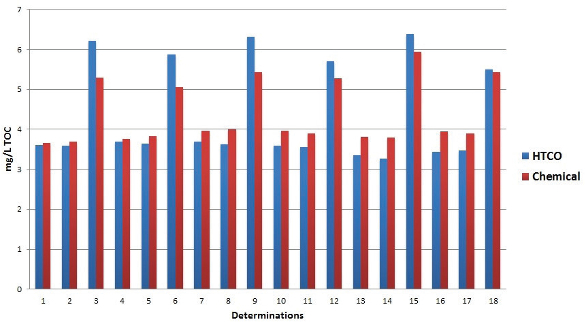
Figure 3 – Comparison of chemical oxidation and HTCO in drinking water samples.
Thus, when HTCO and chemical oxidation results are compared, even though HTCO results trend higher than chemical oxidation, the results always seem to lie within each other’s experimental error. Figure 3 shows a comparison of chemical oxidation and HTCO in drinking water samples. The optimum choice of analyzer should always be made based on the intended application and required sensitivity levels. For lower detection levels, a method utilizing larger sample volumes (chemical oxidation) should be chosen. For carbon levels above 1.0 mg/L, the choice of oxidation technique is not so obvious.
Often mentioned but rarely defined, difficult-to-optimize compounds include humic acids, cellulose, alkaloids, large-chain surfactants and bacteria. If these compounds are known to exist in concentrations greater than 1.0 mg/L, then HTCO is the obvious choice of analyzer. If, however, they exist at lower concentrations, then partial recovery is better than no detection at all and a chemical oxidation method should be used.
References
- Menzel, D.W. and Vacarro, R.F. The measurement of dissolved organic and particulate carbon in seawater.Limnology and Oceanography 1964, 9, 138–42.
- Wilson, R.F. Measurement of organic carbon in seawater. Limnology and Oceanography 1961, 6, 259–61.
- Ehrhard, M. A new method for the automatic measurement of dissolved organic carbon in sea water. Deep Sea Research and Oceanography Abstracts 1969, 16(4), 393–7).
- Cauwet, G. Automatic determination of dissolved organic carbon in seawater in the sub-ppm range. Marine Chemistry June 1984, 14(4), 297–306.
- Sugimara, Y. and Suzuki, Y. A high-temperature catalytic oxidation method for the determination of non-volatile dissolved organic carbon in seawater by direct injection of a liquid sample. Marine Chemistry June 1988, 24, 105–31).
