Introduction
Nowadays, the cultivation of grapes is widely spread around the world with an estimated surface area of 7.6 million of hectares in 2014 [1]. Grape production is an important activity due to the high nutritional properties of grapes and their ancient domestication leading to a large variety of by-products [2]. Grapes are consumed both as fresh and as processed products such as wine, jam, juice, jelly, grape seed extract, raisins, vinegar and grape seed oil. In 2014 the global grape production was estimated at 73.7 million tons. From this, 41% was produced in Europe, 29% in Asia and 21% in America. Approximately 45% of the grape production consists of unpressed grapes, while the other 55% is mainly used for wine production. Up to 78% (24.8 million tons in 2014) of the unpressed grapes is consumed as fresh grapes [1]. All this shows that the grape market plays a very important role in the world food consumption.
In grape production, pesticides are used to control pests and diseases in vineyards to increase crop yield. The most common fungal diseases in vineyards are powdery mildew (Uncinula necator), downy mildew (Plasmopora viticola) and gray mold (Botrytis cinerea)[3]. The most menacing insects in grape plants are the European grapevine moth (Lobesia botrana), vine mealybug (Planococcus ficus), and the citrus mealybug (Planoccoccus citri) [4]. To prevent these, a large variety of pesticides, especially fungicides and insecticides, are applied frequently during the cultivation of grapes (Table 1). In some cases, unsuitable agricultural practices are used during the application of these active materials in the vineyard. As a result the level of pesticide residues in or on grapes at the moment of harvest is higher than the permitted level by regulation [5]. Apart from the environmental risk, a high level of pesticide residues can affect the quality of the grapes and its processed products and it may ultimately reach the consumer and cause health hazards. Therefore, in order to prevent health risks it is important to monitor the presence of pesticides and regulate their levels in grapes. In the European Union, Regulation 396/2005/EC establishes the maximum residue levels (MRLs) of pesticides permitted in products of animal or vegetable origin intended for human or animal consumption [6]. The MRLs for pesticide residues in grapes mostly range between 0.01 mg/kg and 5 mg/kg depending on the pesticide, but in some cases higher limits are established, e.g., for fosetyl-aluminium 100 mg/kg [6].
Most common pesticides used in vineyards.
a: MRL defined as benalaxyl including other mixtures of constituent isomers including benalaxyl-M (sum of isomers).
b: MRL defined as carbendazim and benomyl (sum of benomyl and carbandazim expressed as carbendazim) (R).
c: MRL defined as dimethoate (sum of dimethoate and omethoate expressed as dimethoate).
d: MRL defined as dimethomorph (sum of isomers).
e: MRL defined as fenthion (fenthion and its oxygen analogue, their sulfoxides and sulfone expressed as parent) (F).
f: MRL defined as indoxacarb (sum of indoxacarb and its R enantiomer).
g: MRL defined as malathion (sum of malathion and malaoxon expressed as malathion).
h: MRL defined as metalaxyl and metalaxyl-M (metalaxyl including other mixtures of constituent isomers including metalaxyl –M (sum of isomers)).
i: MRL defined as parathion-methyl (sum of parathion-methyl and paraoxon-methyl expressed as parathion-methyl).
| Pesticide | Family-activity | Pest control | MRL Table/Wine grapes (mg/kg) |
|---|---|---|---|
| Acetamiprid | Neonicotinoid insecticide | Leafhoppers and other small insect pests | 0.5 |
| Azinphos-methyl | Organothiophosphate acaricide/insecticide | Insect and mite pests | 0.05 |
| Azoxystrobin | Strobilurin fungicide | Downy mildewPhomopsis cane and leaf spotPowdery mildewRotbrenner | 2 |
| Benalaxyl | Anilide fungicide | Downy mildew | 0.3a |
| Benalaxyl-M (or Kiralaxyl) | Anilide fungicide | Downy mildew | 0.3a |
| Bifenthrin | Pyrethroid insecticide | Insect and mite pests | 0.2 |
| Boscalid | Anilide-pyridine fungicide | Grey mouldPowdery mildew | 5 |
| Bromopropylate | Diphenyl acaricide | Mite pest | 0.01 |
| Captan | Phatalamide fungicide | 0.02 | |
| Carbaryl | Carbamate acaricide/insecticide | Grape leaffolder and leafrollerGrape berry moth | 0.01 |
| Carbendazim | Benzimidazole fungicide | Broad-spectrum of fungi diseases | 0.3/0.5b |
| Chlorothalonil | Aromatic fungicide | Downy mildew | 3 |
| Chlorpyrifos | Organothiophosphate acaricide/insecticide | European grapevine mothVine and citrus mealybugs | 0.5 |
| Chlorpyrifos-methyl | Organothiophosphate insecticide | Grape mothVine and citrus mealybugs | 0.2 |
| Cyazofamid | Imidazole-sulfonamide fungicide | Downy mildew | 0.5 |
| Cyfluthrin | Pyrethroid insecticide | 0.3 | |
| ?-Cyhalothrin | Pyrethroid insecticide | Insect and mite pests | 0.2 |
| Cymoxanil | Aliphatic nitrogen fungicide | Downy mildew | 0.2 |
| Cypermethrin | Pyrethroid insecticide | Insect and mite pests | 0.5 |
| Cyprodinil | Anilinopyrimidine fungicide | Grey mould | 5 |
| Deltamethrin | Pyrethroid insecticide | Insect and mite pests | 0.2 |
| Dichlofluanid | Phenyldulfamide fungicide/acaricide | Downy mildew | 0.01 |
| Dimethoate | Organothiophosphate acaricide/insecticide | Vine and citrus mealybugs | 0.02c |
| Dimethomorph | Morpholine fungicide | Downy mildew | 3d |
| Endosulfan | Organochlorine insecticide | Insect and mite pests | 0.05 |
| Famoxadone | Dicarboximide-oxazole fungicide | Downy mildew | 2 |
| Fenamidone | Imidazole fungicide | Downy mildew | 0.5 |
| Fenarimol | Pyrimidine fungicide | Broad-spectrum of fungi diseases | 0.3 |
| Fenhexamid | Anilide fungicide | Grey mould | 5 |
| Fenitrothion | Organothiophosphate insecticide | 0.01 | |
| Fenpropathrin | Pyrethroid insecticide | Insect and mite pests | 0.2 |
| Fenthion | Organothiophosphate insecticide | 0.01e | |
| Fluazinam | Pyridine fungicide | Grey mould | 0.05/3 |
| Flusilazole | Conazole fungicide | Botrytis | 0.01 |
| Fludioxonil | Pyrrole fungicide | Grey mould | 4-May |
| Flufenoxuron | Benzoylphenylurea chitin synthesis inhibitors insecticide/acaricide | Grape moth | 2-Jan |
| Fluquinconazole | Conazole fungicide | Foliar fungi and rust diseases | 0.1/0.5 |
| Folpet | Phthalimide fungicide | Downy mildewPhomopsis cane and leaf spotPowdery mildewRotbrenner | 0.02/10 |
| Hexythiazox | Thiazolidine acaricide | Mite growt regulator | 1 |
| Imazalil | Conazole fungicide | Prevent fruit fungi diseases in transport and storage | 0.05 |
| Imidacloprid | Neonicotinoid insecticide | Grape mothVine and citrus mealybugs | 1 |
| Indoxacarb | Carbamate insecticide | Grape moth | 2f |
| Iprodione | Imidazol fungicide | Grey mold | 10 |
| Iprovalicarb | Carabamate fungicide | Downey mildew | 2 |
| Kresoxim-methyl | Strobilurin fungicide | Powdery mildew | 1 |
| Lufenuron | Benzoylphenylurea chitin synthesis inhibitors insecticide | Grape moth | 1 |
| Malathion | Organothiophosphate acaricide/insecticide | 0.02g | |
| Mandipropamid | Amide fungicide | Downy mildew | 2 |
| Maneb-group | Dithiocarbamate fungicide | 5 | |
| Mepanipyrim | Anilinopyrimidine fungicide | Grey mold | 2 |
| Metalaxyl | Anilide fungicide | Downy mildew | 2/1h |
| Methidathion | Organothiophosphate insecticide | 0.02 | |
| Methomyl | Oxime carbamate insecticide | Insect and mite pests | 0.02/0.5 |
| Methoxyfenozide | Moulting hormone agonist | Lepidoptera pest | 1 |
| Metrafenone | Aryl phenyl ketone fungicide | Powdery mildew | 5 |
| Myclobutanil | Conazole fungicide | Powdery mildew | 1 |
| Omethoate | Organothiophosphate insecticide/acaricide | Insect and mite pests | 0.02 |
| Parathion-methyl | Organothiophosphate insecticide | 0.01i | |
| Penconazole | Conazole fungicide | Powdery mildew | 0.2 |
| Phosalone | Organothiophosphate acaricide/insecticide | European grapevine moths | 0.01 |
| Procymidone | Dichlorophenyl dicarboximide fungicide | Grey mold | 0.01 |
| Profenofos | Organothiophosphate insecticide | Insect pest | 0.01 |
| Propiconazole | Conazole fungicide | Powdery mildew | 0.3 |
| Proquinazid | Unclassified fungicide | Powdery mildew | 0.5 |
| Pyraclostrobin | Strobilurin fungicide | Broad-spectrum of fungi diseases | 2-Jan |
| Pyrimethanil | Anilinopyrimidine fungicide | Grey mould | 5 |
| Quinalphos | Organothiophosphate acaricide/insecticide | 0.05 | |
| Quinoxyfen | Quinoline fungicide | Powdery mildew | 1 |
| Spiroxamine | Unclassified fungicide | Powdery mildew | 1 |
| Tebuconazole | Conazole fungicide | Powdery mildew | 0.5/1 |
| Tebufenozide | Moulting hormone agonist insecticide | Grape moth | 3 |
| Tetraconazole | Conazole fungicide | Powdery mildew | 0.5 |
| Thiabendazole | Benzimidazole-thiazole fungicide | Prevent fruit fungi diseases in transport and storage | 0.05 |
| Thiophanate methyl | Carbamate fungicide | 0.1/3 | |
| Triadimefon | Conazole fungicide | 2 | |
| Trifloxystrobin | Strobilurin fungicide | Black rotDowny mildewPowdery mildew | 5 |
| Valifenalate | Acylamino acid fungicide | Downy mildew | 0.2 |
| Vinclozolin | Oxazole fungicide | Grey mold | 0.05 |
| Zoxamide | Benzamide fungicide | Downy mildew | 5 |
To measure these low concentrations highly selective, sensitive and accurate analytical methods are needed. Due to the large number of pesticides on the market, the use of multi-residue methods capable of analysing large numbers of pesticides in one single run is the most common and most efficient approach. In the European Union (EU) a joint work of European Union Reference Laboratories (EURLs) and National Reference Laboratories (NRLs) of each EU member state maintain and improve the quality, accuracy and comparability of the measurements and results between Official Laboratories. The EURLs are responsible for guiding and providing analytical methods, organising proficiency tests, and promoting the development and validation of new analytical methods. The EURL for pesticides in fruit and vegetables (EURL-FV) published on its website a multi-residue method called the Mini-Luke sample extraction, which is based on the original Luke method [7]. This analytical method has recently been improved, validated and implemented in routine in the Dutch NRL by Lozano et al. [8]. Also other organizations as the European Committee for Standardization (CEN) assist laboratories by providing some standard methods for the determination of pesticide residues in foods of plant origin. In 2008, three analytical multi-residue methods based on gas chromatography coupled to mass spectrometry (GC–MS) [9] and [10] and/or liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) [10] and [11] were published, where grapes were tested as one of the representative fruit matrices. The Association of Official Analytical Chemists (AOAC) International also published an official method for the analysis of pesticide residues in representative matrices as grapes, lettuces and oranges, with a common sample treatment followed by GC–MS and LC–MS/MS analysis [12]. Besides the use of official methods, many laboratories develop and validate their own method for pesticide residues analysis because depending on the analytical technique chosen, different approaches for sample treatment may be considered. Even when using the same technique, different equipment or equipment settings can be selected, making it difficult to reach a universally accepted analytical method. The European Commission’s Directorate for Health and Food Safety (DG SANTE) provides guidance to laboratories for the validation of methods for pesticide residues analysis in food and feed. [13] This guidance allows the laboratories to have free choice of methods, which is beneficial for the continuous development of the analytical methods. Laboratories performing analyses of pesticide residues also tend to work under a quality system like ISO/EC 17025 [14] to ensure a consistent and reliable approach with the use of quality control measures like certified reference materials and participation in proficiency tests [15] and [16].
This paper presents an overview of the evolution in analytical methods for pesticide residue analysis in grapes during the last decade. By illustrating the large variety of pesticides occurring in the vineyard, it aims to explain the large range of analytical methods developed for the analysis of pesticides in grapes until today. The review focuses on the limitations of these methods and on potential future perspectives.
Use and occurrence of pesticides in grape cultivation
According to the principles of integrated pest management the monitoring of pesticide residues is essential in order to predict the proper concentrations and number of applications of pesticides needed and to determine the pre-harvest interval. The application of the principles of integrated pest management and good agricultural practices resulted in a reduction of pesticide usage with the tendency to reduce the most environmental dangerous pesticides [17]. Because of this the number of common pesticides applied and found as residues at harvest is normally lower than the number of pesticides registered by the relevant authorities in each country (Table 1). For instance, there are around 450 pesticides in the EU database [6] and [18] for which the MRLs have been established in table and wine grapes, but according to the literature less than half of them are actually applied for pest control in vineyards (see Table 1 for common pesticides and MRL values in Europe). Another example can be found in the common integrated pest management guidelines for grapes where the number and the quantity of broad-spectrum organophosphate and carbamate products dropped considerably [19].
A number of studies dealing with the monitoring of pesticides in grapes have been published. A study by Česnik et al. [20] in which pesticide residues in wine grapes were monitored in three different regions in Slovenia showed that the most frequently found pesticides in grapes were folpet (97,9%), cyprodinil (51.1%), dithiocarbamates (44.7%), chlorothalonil (23.4%), chlorpyriphos (19.1%) and pyrimethanil (14.9%). The concentration range of these pesticide residues found in grapes were below the MRLs described in the EU regulation [4], except in case of cyprodinil and fludioxonil which exceeded the MRL in 38,3 % of the samples. Two surveys [21] and [22] for table grapes carried out in three different regions in Turkey showed that chlorpyrifos-methyl and chlorpyrifos-ethyl, besides deltamethrin and λ-cyhalothrin, were the most frequently found pesticides [21]. Moreover the pesticides azoxystrobin, boscalid, cyprodinil, dimethomorph, flufenoxuron, hexythiazox, imazalil, methomyl, penconazole and thiophonate methyl were detected in concentrations above the MRLs [22]. In a survey of the Egyptian market [23] the most detected pesticides in grapes were carbendazim, acetamiprid, boscalid, λ-cyhalothrin, profenofos and pyraclostrobin. Other frequently found pesticides in grapes were cyprodinil, chlorpyrifos, delthamethrin and iprodione. An exhaustive analysis carried out during 1998 and 2003 by the National Food Institute in Denmark on imported grapes from 17 different countries (considering as main exporters Italy and South Africa) [24] concluded that some samples from Italy exceeded the MRLs for the pesticides phosalone, fenitrothion and bromopropylate, while samples from South Africa had residues of prothiofos. Another study in fruits and vegetables reported the presence of the pesticides captan and methomyl at concentrations higher than the MRL in table grapes from Chile [25]. A recent study carried out in La Rioja region in Spain monitored the pesticides in the soils of seventeen vineyards. The highest concentrations were found for the fungicides metalaxyl and triadimenol, the herbicides fluometuron and terbuthylazine and the insecticide methoxyfenozide [26].
Four studies carried out in different areas of India examined the persistence of the pesticides azoxystrobin [27], fluopicolide [28], tebuconazole [29] and kresoxim methyl [30] in grapes. In each study one pesticide was applied to the grapes. All studies concluded that the residue of the pesticide was below the quantifiable limit (azoxystrobin, fluopicolide, tebuconazole) or well below the EU MRL (kresoxim methyl) at the time of harvest when grapes were treated with the recommended dose of pesticide and the pre-harvest interval was respected.
Other interesting studies on grapes deal with the potential variability in the levels of pesticide residues in single grapes [31], depending on the growth conditions, the different localisations (grape peel or pulp) and the different modes of action [32]. A study carried out with the pesticides acetamiprid and cypermethrin in grapes concluded that the distribution patterns of both pesticide residues were influenced by complex factors such as differences in crop species, plant cultivation methods, application rates, pre-harvest intervals and physicochemical properties of pesticides [31]. In another study [32], fourteen pesticides (13 fungicides and 1 insecticide) were selected to investigate the mobility from peel to pulp in grapes, considering lipophilicity and concentration of the active ingredients as the essential parameters for residue transfer from peel to pulp. The results obtained were difficult to interpret: most systemic pesticides such as cymoxanil and oxadixyl were found in the pulp, while only the contact pesticide folpet was detected in the peel and not in the whole grape. The removal of pesticides from grapes by washing did not exceed 70%, but it could be concluded that consumer intake of pesticides from grapes significantly decreased as a result of water washing [32]. In reference [33], a multi-residue method for the analysis of 175 pesticides was used to investigate the peel and pulp distribution ratio of 25 pesticides detected in grape samples. Four groups of pesticides were distinguished depending on their distribution between peel and the whole grape. The first group with a peel/whole grape distribution of 100%, meaning that the pesticides were exclusively located in the peel, consisted of pesticides with a strong lipid solubility (fenvalerate, p,p’-DDE, chlorpyrifos, cypermethrin, cyhalothrin, pyridaben, chlorfenapyr or bifenthrin). A second group had a peel/whole grape distribution of 80–99.9% (difenoconazole, pyraclostrobin, famoxadone, prochloraz, hexaconazole, chlorothalonil, flusilazole, azoxystrobin and iprodione). A third group of pesticides with a 50–80% of peel/whole grape distribution showed a 20–50% migration into the pulp (dimethomorph, cyprodinil, tebuconazole, propiconazole, kresoxim-methyl and procymidone). In case of the fourth group (0–50% peel/whole grape distribution) more than half of the pesticide residue can migrate into the pulp (pyrimethanil and metalaxyl). As the main part of the pesticide residues of the first and second group can be removed by peeling or washing, these pesticides can be recommended for grapes cultivation based on their distribution pattern, while the third and fourth group should not be recommended for grapes cultivation [33]. In a recent work from Lagunas-Allué et al. [34], the mobility and distribution of eight fungicides (vinclozolin, dichlofluamid, captan, penconazol, quinoxyfen, fluquinconazol, boscalid, pyraclostrobin) in surface, skin and pulp of grapes was studied. One of the most interesting outcomes was that the sorption of the fungicide did not depend on the initial spiked concentration but on the time that grapes had been in contact with the fungicide solution. Although all fungicides showed penetration into the pulp, residues were mainly found in the skin. In this study, pyraclostrobin showed a higher penetration than the other fungicides.
In many cases grapes are processed in order to make other products, and then it is possible that residues of pesticides pass from grapes to those products. For instance in wine processing, pesticide residues in grapes may transfer to the must and influence the selection and development of yeast strains [4]. In these contexts, a high number of analytical studies has focussed on the dissipation rates and/or concentration factors of pesticides in different parts of the derived products during the grapes processing, like drying, juice/wine-making, alcoholic beverage distillation, food supplements extracts or pharmaceutical/cosmetic applications. The dissipation rate describes the dissipation kinetics of the pesticide in grapes which often follows a first-order model. It is used to calculate the pre-harvest interval, which is the time period (in days) required for dissipation of the initial residue deposits to below the MRL, and the half life, t1/2, which is the time at which the concentration of initial deposits reaches the 50% level. [35] and [36] In a review of P. Cabras and A. Angioni [37], 9 fungicides and 9 insecticides residues in grapes were monitored at 5 harvest intervals, resulting in different decay rates till dissipation 21 or 28 days after application for most of them. In this study, it was shown that penconazole, fluazinam, kresoxim-methyl and organophosphorous insecticides disappear quickly from the grapes after treatment, whereas the fungicides fludioxonil and pyrimethanil showed a slower decay rate (half life, t1/2,of 24 and 57 days, respectively) and were detectable at harvest time. For pyrimethanil this might be explained by its migration into the pulp, as shown in reference [33]. During the drying process for raisins production the residues level could theoretically increase by a factor of 4. However, for seven monitored pesticide residues (benalaxyl, dimethoate, iprodione, metalaxyl, phosalone, procymidone and vinclozolin) the values of concentration decreased for all except iprodione and phosalone which showed a higher concentration (factor of 1.6 and 2.8, respectively). The same study showed that in the case of wine production, pesticide residues (13 fungicides and 9 insecticides) were distributed over a biphasic system made up of a liquid phase (the must) and a solid phase (cake and lees) after pressing of the grapes. In general pesticide residues in the must were remarkably lower than those on the grapes showing the great affinity of most pesticides for the solid phase. After fermentation, pesticide residue levels in wine were always lower than those on the grapes and in the must; the only exception were those pesticides which preferentially partition in the liquid phase (azoxystrobin, dimethoate and pyrimethanil). These pesticides were present in the wine at the same concentration as in grapes. In case of alcoholic beverages derived from wine by-products, only fenthion, quinalphos and vinclozolin pass into the distillate from the lees when present in very high concentrations [37]. Other studies of pesticide residues dissipation in wine have been carried out [38], [39], [40], [41], [42], [43], [44], [45], [46] and [47], and dissipation rates were estimated for different compounds. However, this review article has not as a purpose to go into detail in this wine production process.
Analytical methods
According to the guidelines given by the European Commission’s Directorate for Health and Food Safety (DG SANTE), grapes have been classified in the commodity group of ‘high acid content’ and ‘high water content’ together with small fruit and berries [13]. However, grapes are often considered as a medium acid matrix with a high sugar content when multi-residue methods for pesticide analysis in fruit and vegetables are developed [5] and [48]. Therefore these multi-residue methods used for the analysis of pesticides in grapes often follow the general strategies for pesticide analysis in fruits and vegetables, with a common extraction step and clean-up followed by chromatography and MS detection.
During this review, the references were separated in two groups using two criteria: first, the number of pesticides included in the method, and second the matrices analyzed by the method. The methods including a large number of pesticides from different families were considered as multi-residue methods. These are commonly applied to different matrices including grapes. They are presented in Table 2[5], [33], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71] and [72]. The methods focussing on the matrix grape and not including a large number of pesticides were considered as single-residue methods or specific methods for grapes. These methods often include some of the by-products of grapes as additional matrices. They are presented in Table 3[32], [38], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97] and [98]. In both Table 2 and Table 3, the papers are classified in a chronological order to outline the evolution of the analytical methodologies.
Overview of published multi-residue methods for the analysis of pesticides in grapes. Recovery is expressed in percentage between the theoretical value and the experimental. Repeatability is expressed as relative standard deviation percentage.
a: Sensitivity defined as LOQ as the lowest level assayed during validation when the LOD or other LOQ estimation is provided in the paper.
b: Validation of the method in cabbage and apple.
| Number of analytes | Sample treatment | Determination technique | Trueness (mg/kg) | Recovery (%) | Repeatability (%) | Sensitivity LOD (mg/kg) 103 | Reference |
|---|---|---|---|---|---|---|---|
| 38 | -SLE: 8 g sample + 50 mL ethyl acetate + 70 g Na2SO4 + 2 g NaHCO3-Low volume evaporation: 2 × (10 mL methanol to 2 mL final volume) | LC-ESI-MS/MS (QqQ) | 0.010.8 | 7392 | 4.517.7 | 1a | [49] |
| 57 | -SLE: 75 g sample + 200 mL ethyl acetate + 40 g Na2SO4 + (NaOH for acid matrices)-Dryness evaporation of aliquot and dissolved in methanol | LC-ESI-MS/MS (QqQ) | 0.010.5 | 44118 | 330 | 1a | [50] |
| 74 | -SLE: 20 g sample + 10 mL water + pH adjustment 67 + 40 mL ethyl acetate-Dryness evaporation aliquot of 5 mL and dissolved in 1 mL methanol | LC-ESI-MS/MS (QqQ) | 0.011 | 63158 | 331 | 1a | [51] |
| 446 | -SLE: 20 g sample + 40 mL acetonitrile + 5 g NaCl-SPE: Envi-18 elution acetonitrile + evaporation 1 mLSPE: Envi-Carb connected to aminopropyl Sep-Pak | GCMS (Q)LC-ESI-MS/MS (QqQ) | 0.013.00 | 55134 | 2.139.1 | 0.525 (LOD) | [52] |
| 309 | -SLE: 75 g sample + 200 mL ethyl acetate + 15 g NaHCO3 + 40 g Na2SO4-Dryness evaporation 100 mL and dissolve in 5 mL ethyl acetate: cyclohexane (1:1 v/v)-Dilute 5 times with ethyl acetate: cyclohexane (1:1 v/v) for GC-Dryness evaporation aliquot 0.5 mL an | GCMS/MS (QqQ)LC-ESIMS/MS (QTrap) | 0.010.05 | 57122 | 230 | 1a | [53] |
| 82 | -SLE: 10 g sample + 10 mL ethyl acetate + 10 g Na2SO4-dSPE: aliquot 5 mL + 25 mg PSA-Clean aliquot of 4 mL + 200 L 10% diethylene glycol in methanol-Dryness evaporation and dissolution in 2 mL methanol:water 0.1% acetic acid 1:1 v/v | LCMS/MS (QTrap) | 0.00250.05 | 36.5120.5 | 0.319 | 0.13.3 | [54] |
| 341 | -SLE: 25 g sample + 40 mL ethyl acetate + 25 g Na2SO4-For GC | GCMS (Q)LC-ESI-MS/MS (QqQ) | 0.0010.5 | 60140 | 1530 | 1 for most pesticides | [55] |
| 171 | -SLE: 157.5 g sample + 30 mL acetone + 30 mL dichloromethane + 30 mL light petroleum (+Na2SO4)-Dryness evaporation 1.1 mL aliquot | LCMS/MS (QqQ) | 0.010.1 | 21114 | 150 | 10a | [56] |
| 80 | -QuEChERS: 10 g sample + 10 mL acetonitrile + 4 g MgSO4 + 1 g NaCl + 0.5 g disodiumhydrogen citrate sesquihydrate + 1 g trisodiumcitrate dehydrate-dSPE: aliquot extract + 150 mg MgSO4 + 25 mg PSA/mL extract-Re-acidify extract: 10 L formic acid 5% (v/v)/m | GCMS/MS (QqQ)LCMS/MS (QTrap) | 0.0050.2 | 60127 | 0.216.7 | 10a | [48] |
| 151 | -QuEChERS: 10 g sample + 10 mL acetonitrile + 4 g MgSO4 + 1 g NaCl + 1 g citrate dehydrate + 0.5 g di-sodium hydrogen citrate sesquihydrate-dSPE: 6 mL aliquot extract + 150 mg PSA + 950 mg MgSO4-Acidification before LC injection: 1.5 mL extract + 15 L 5% | GCMS (Q)LCMS/MS (IT) | 0.050.5 | 33120 | 0.714.5 | 0.4115 g/kg | [57] |
| 51 | -SLE: 10 g sample + 10 mL ethyl acetate + 10 g Na2SO4-dSPE: 1 mL extract + 25 mg PSA | GCxGCMS (TOF) | 0.01 | 70109 | 310 | 0.23.0 | [58] |
| 38 | -MSPD: 500 mg sample + 500 mg C8 + 700 L elution ethyl acetate-Dryness evaporation of extract and dissolution isooctane | GCMS (Q)GCxGC-ECD | 0.5 | 62102 | 121 | 9250 GCMS; 0.0053.6 GCxGC-ECD | [59] |
| 160 | -SLE.: 10 g sample + 10 mL ethyl acetate + 10 g Na2SO4-dSPE: 1 mL aliquot + 25 mg PSA | GCxGCMS (TOF) | 0.010.05 | 67135 | 112 | | [60] |
| 346 | -SLE: 15/20 g sample + 15/40 mL acetonitrile + 6 g MgSO4 + 1.5 g NaCl- Method A: dSPE: extract + 0.3 g PSA + 1.8 g MgSO4-Method B: SPE: Envi-18 | GCMS (Q) | 0.010.2 | 30136 | 1020 | 1.7266 | [61] |
| 50 | -SLE: 10 g sample + pH ajustment 4 acetic acid + 10 mL ethyl acetate + 10 g Na2SO4 + 5 mL ice cold water-dSPE: 1 mL extract + 25 mg PSA | GCMS/MS (QTrap) | 0.010.05 | 71117 | 318 | 5.019.2 | [62] |
| 150 | -SLE: 10 g sample + 10 mL acetonitrile + 4 g MgSO4 + 1 g NaCl + 1 g trisodium citrate dehydrate + 5 g disodium hydrogencitrate sesquihydrate-Dryness evaporation 4 mL aliquot | LCMS/MS (QTrap) | 0.010.1 | 40109 | 125 | 10a | [63] |
| 135 | -SLE grape: 10 g sample + 10 mL ethyl acetate + 10 g Na2SO4;-dSPE: 1 + mL extract + 25 mg PSA | GCMS (TOF) | 0.0010.650 | 70120 | 132 | 0.030.38 | [64] |
| 209 | -SLE: 10 g sample + 10 mL acetonitrile + 4 g MgSO4 + 1 g NaCl-dSPE: ca. 9 mL extract + 400 mg PSA + 1200 mg MgSO4 | LCMS/MS (QTrap) | 0.010.5 | 77121 | 933 | 0.110 | [65] |
| 82 | -SLE: 15 g sample + 15 mL acetonitrile + 6 g MgSO4 + 1.5 g NaOAc + 0.1% acetic acid-Dryness evaporation 10 mL | GC-NCI-MS/MS (QqQ) | 0.010.02b | 58.7124.4b | 3.915.9b | 0.011.82 | [66] |
| 175 | -SLE: 2.5 g peel/5 g pulp + 20 mL acetonitrile + 1 g NaCl + 4 g MgSO4-dSPE: 5 mL extract + 250 mg MgSO4 + 60 mg PSA-Dryness evaporation 4 mL extract dissolved 1 mL cyclohexane:acetone (7:3 | GPC-GCMS (Q) | 0.010.2 | 46.5145 | 2.034.6 | 0.410 | [33] |
| 48 | -SLE: 0.5 g sample + 900 L acetonitrile. Vortex + Ultrasonic bath-On-line chromatographic cleanup | LCMS/MS (QqQ) | 0.010.25 | 64121 | 420 | 0.810.3 | [67] |
| 349 | -SLE: 10 g sample + 10 mL ethyl acetate + 10 g Na2SO4-dSPE: 1 mL extract + 25 mg PSA + 7 mg GCB-Dryness evaporation and dissolution 800 L ethyl acetate | GCMS/MS (QqQ) | 0.0050.025 | 70120 | <20 | 510 | [68] |
| 71 | -Pressurised liquid solvent extraction: 10 g sample + 1.0 mL tetrafluoroethane-toluene = 100 mbar pressure + 15 mL 1 | UHPLCMS/MS (QqQ) | 0.0010.01 | 70.8119.1 | <20 | 0.122.16 | [5] |
| 166 | -SLE: 15 g sample + 15 mL acetonitrile 1% acetic acid + 1.5 g NaOAc + 6 g MgSO4-dSPE: extract + 900 mg MgSO4 + 150 mg C18 + 300 mg PSA-Evaporation to 0.10.2 mL | UHPLC-ESIMS/MS (QOrbitrap) | 0.01 0.4 | 42.9123.8 | 5.228.3 | <5 | [69] |
| 47 | -SLE: 10 g sample + 10 mL ethyl acetate + 10 g Na2SO4-dSPE: 1 mL extract + 25 mg PSA | GCMS (Q) | 0.010.02 | 67120 | 119 | <0.010.02 mg/kg LOQ | [70] |
| 341 | -SLE: 10 g sample + 10 mL ethyl acetate + 10 g Na2SO4-dSPE: 1 mL + 25 mg PSA + 7 mg GCB | GCMS/MS (QqQ) | | | | | [71] |
| 60 | -SLE: 10 g sample + 25 mL acetonitrile:methanol (90:10 | UHPLCMS (TOF) | 0.050.2 | 73111 | 14 | 0.811.8 | [72] |
Overview of published specific methods for the analysis of pesticides in grapes and derived products. Recovery is expressed in percentage between the theoretical value and the experimental. Repeatability is expressed as relative standard deviation percentage.
| Number of analytes | Matrix | Sample treatment | Determination technique | Trueness (mg/kg) | Recovery (%) | Repeatability (%) | Sensitivity LOD (mg/kg) 103 | Reference |
|---|---|---|---|---|---|---|---|---|
| 12 multiclass fungicides | GrapeMustWine | -SLE(1): 5g/50 mL sample + 30 mL acetone:dichloromethane (1:1 v/v) + 2g NaCl (Probe blender)-Dryness evaporation + 5 mL isooctane-toluene (1:1 | GC-ECDGC-NPDGC-EI-MS (Q) | 0.010.5 | 78107 | 17.50.6 | 0.775.16 | [73] |
| 15 multiclass pesticides | Skin/Whole grape | -SLE: 10025 g sample + 10025 mL methanol-Dryness evaporation and dilution in 25 mL water:methanol (88:12 v/v)-SPE: 500 mg C8 | LC-DAD | | 30.779.4 | 19.239.4 | 1.7 average | [32] |
| 12 botanical insecticides | Grape | -SLE: 5 g sample + 10 mL acetonitrile + 4 g NaCl + 1 g MgSO4 | LC-DADLC-APCI-MS | 0.015 | 73115 | 0.112.2 | 0.10.01 | [74] |
| 6 multiclass fungicides | GrapeWine | -SLE/LLE: 5 g/5 mL + 10 mL ethyl acetate:hexane (1:1 v/v)-Dryness evaporation of 1 mL and disolved with 1 mL of methanol:water (80:20 v/v) for LC and 0.5 mL of Ethyl acetate:hexane (1:1 v/v) | LC-DADGCMS(Q) | 0.252.00 | 96105 | 612 | 0.10.3 | [75] |
| 10 multiclass pesticides | Grape | -SLE: 50 g sample + 100 mL ethyl acetate + 75 g Na2SO4-Dryness evaporation of 2 mL aliquot and dissolved with 0.45 mL methanol | LCMS/MS (QqQ) | 0.010.1 | 78104 | 615 | 510 | [76] |
| 3 multiclass fungicides | GrapeWine | -SLE: 10 g/10 mL sample + 10 mLcyclohexane:dichloromethane (9:1 | GC-NPDGC-ECDGCMS/MS (IT) | 0.052.0 | 81102 | 312 | 550 GC-ECD; 10100 GC-NPD | [77] |
| 8 organophosphorus pesticides | Grape juice | -Dilution juice: 10 mL sample + 10 mL MilliQ water | GC-NPD | 0.151.35 | 75103 | 1.96.3 | 1.857.32 | [78] |
| 18 multiclass pesticides | GrapeMustWineVinegard | -SLE: 10 g sample + 10 mL methanol (UAE)-SBSE: 20 mm x 0.5 mm PDMS 1000 rpm 25 °C 150 min | GCMS (Q) | 72122 | 320 | 6.740.0 | [79] | |
| 27 multiclass pesticides | GrapeMustWine | -QuEChERS: 10 g sample + 10 mL acetonitrile + 4 g MgSO4 + 1 g NaCl manual shaking and centrifugation-dSPE: 1 mL aliquot extract + 150 mg MgSO4 + 50 mg PSA + 50 mg C18 | LP-GCMS (Q) | 0.045 | 57120; 63120; 52121 | 520; 317; 318 | 1.012.5; 1.214.0; 1.319 ng/g | [80] |
| 11 fungicides | White/Red GrapeWhite/Red Wine | -SLE.: 15 g/15 mL sample + 15 mL ethyl acetate: hexane (1:1 v/v) Ultrasound bath 10 min. + 1 g NaCl + 5 g Na2SO4-Dryness evaporation 12 mL aliquot dissolution 3 mL acetonitrile-SPE: envi Carb II/PSA | GCMS (IT) | 0.050.5 | 76147 | 216 | <124 | [81] |
| 19 fungicides | GrapeSeed oilMeal grape | -SLE: 15 g grape + 200 mL acetone-LLE partition: 650 mL saturated Na2SO4 | GC-NPDGC-ECDLCMS/MS (QqQ) | 0.051.2 | 7992 GC; 5191 LC | [82] | ||
| 8 multiclass pesticides | Grape | -SLE: 1 g sample + 5 mL acetonitrile + 2 g MgSO4 + 0.5 g NCl + 0.5 g sodium citrate + 0.25 g sodium hydrogenitrate sesquihydrate-DLLME: 88 mg [C6MIM][PF6] + 714 L methanol | LC-DAD | 0.0050.5 | 64100 | 1.79.1 | 0.655.44 | [83] |
| 6 pyrethroid pesticides | Grape | -SLE.: 10 g sample + 30 mL acetone-LLE partition: 30 mL dichloromethane + 30 mL light petroleum + 10 g Na2SO4. UltraturraxDryness evaporation dissolution 1.0 mL ethyl acetate | GCxGC-FIDGCxGC-ECD | 0.020.5 | 94113 | 2.618.4 | 36 | [84] |
| 8 fungicides | Red Grape | -MSPD: 0.5 g sample + 1.5 g C18 | GCMS (Q) | 0.010.06 | 76120 | 3.59.0 | 1.02.6 | [85] |
| 21 pyrethroid pesticides | Grape | -SLE.: 10 g sample + 10 mL ethyl acetate + 10 g Na2SO4-dSPE: aliquot 1 mL + 25 mg PSA | (PTV-LVI)-GCMS/MS (IT) | 0.010.05 | 77115 | 1.519.6 | 0.53.2 | [86] |
| 25 multiclass pesticides | Grape | -SLE: 500 g grape + 200 mL deionized water-Hollow fibre sorptive extraction: SiO2 | GCMS (Q) | 0.43.6 | 61108 | 4.012.4 | 212 | [87] |
| 8 fungicides | Grape | -SLE: 2 g sample + hexane:acetone (1:1 | GCMS (Q) | 0.010.05 | 82107 | 28 | 0.71.7 | [88] |
| 7 multiclass pesticides | Grape | -QuEChERS + dilution acetonitrile: 10 mM ammonium formate (1:4 | LCMS (Q) | 0.10.5 | 90104 | 0.34.1 | | [89] |
| 12 plant growth regulators | Grape | -SLE: 5 g sample + 5 mL methanol 1% HCl + 0.5 g ammonium acetate-SPE: Oasis HLB 200 mg | LCMS/MS (QqQ) | 0.010.1 | 78130 | 457 | 1.010.0 | [90] |
| 5 multiclass pesticides | Grape juice | -Microextraction: dynamic single drop in a narrow-bore tube | GC-FID | 0.52 mg/L | 72106 | 17 | 211.2 | [91] |
| 9 organophosphorus pesticides | Grape | -MSPDE: 0.5 g sample + 1.0 g MWCNT blended | LCMS/MS (QqQ) | 0.00050.2 | 71.2102.8 | 1.811.8 | 0.060.15 | [92] |
| 18 multiclass pesticides | Red/Green Grape | -SLE: 10 g sample + 10 mL acetonitrile + 6 g MgSO4 + 1.5 g NaOAc-dSPE: extract + 400 mg PSA + 1200 mg MgSO4-Dryness evaporation extract dissolution 1 mL acetonitrile | LCMS/MS (QqQ) | 0.00010.025 | 97101 | 0.015.21 | 0.0270.087 | [93] |
| 30 multiclass pesticides | Grape | -SLE: 10 g sample + 10 mL acetonitrile + 1 g NaCl + 4 g MgSO4-Reverse-DSPE: 1 mL extract + 10 mg MWCNTs + 150 mg MgSO4 | GCMS-SIM (Q) | 0.020.2 | 75109 | 313 | 215 | [94] |
| 13 multiclass pesticides | GrapeMustWine | -SLE: 10 g sample + 10 mL acetonitrile + 4 g MgSO4 + 1 g NaCl + 0.5 g disodium hydrogencitrate sesquihydrate + 1 g trisodium citrate dehydrate-dSPE: 5 mL extract + 125 mg PSA + 750 mg MgSO4- Dryness evaporation 1 mL extract | LCMS/MS (QqQ) | 0.01 | 7095 | 0.160 | 0.21.2 | [38] |
| 7 strobilurin fungicides | Grape | -5g sample + 2 mL ethanolSupernatant diluted to 14 mL acetate buffer (0.04 M) + NaCl 5%-SBSE: 200 rpm | LC-DAD | 10175 | 89101 | 29 | 0.32.0 | [95] |
| 10 triazole fungicides | Grape | -SPME: PDMS/DVB | GCMS (TOF) | 0.010.5 | 84114 | 1.714.6 | 0.255 | [96] |
| 11 fungicides | Grape bagase | -UAE: 15 min + 2545 °C + 020% NaCl + 0.5 g sample + 5 mL ethyl acetate/hexane:CH3OCH3 (1:1 v/v)/methanol/hexane-PLE: 0.5 g sample + 1 g cleaned sand + 20 mL hexane:CH3OCH3 (1:1 | GCMS (Q)GCMS/MS (QqQ) | 0.11 | 81120 | 5.112 | 0.161.96 | [97] |
| 130 pesticides | Grape seed extracts | -SLE: 2 g sample + 10 mL ethyl acetate + 5 g MgSO4-dSPE: 1.4 mL extract + 50 mg PSA + 50 mg GBC + 50 mg Z Sep+ + 50 mg C18-Dilution extract with ethyl acetate (1:1 | GCMS/MS (QqQ) | 0.010.1 | 60120 | 121 | 0.55 | [98] |
Sampling and sample preparation
This section deals with the sub-sampling in the laboratory and not with field sampling or acceptance sampling. Correct sample preparation techniques and sub-sampling are needed in order to obtain a homogeneous and representative sample. In general, the starting material consists of 0.5 kg–2 kg of grapes [32], [48], [49], [50], [51], [52], [53],[54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [73], [74], [75], [76], [77], [78], [79],[80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96],[97] and [98], which represents the sample arriving in the laboratory for analysis. These are removed from the stems and the whole berries with the peel are blended. In some cases, the grapes are first frozen and the sample is homogenised by cryogenic milling[48]. Once the sample is homogenised a sub-sample, ranging from 0.5 g to 100 g (but typically 10 g) is taken for further extraction and analysis.
Sample extraction
The complexity of the sample treatment is linked to the potential matrix interferences and the used separation technique, most commonly GC [48], [52], [53], [55], [57], [58], [59],[60], [61], [62], [64], [66], [68], [70] and [71] or LC [5], [48], [49], [50], [51], [52], [53], [54],[55], [56], [57], [63], [65], [67], [69] and [72]. Also the physicochemical properties of the analyte, mainly the polarity of the pesticide, have to be considered. An evolution in extraction methods together with the parallel improvement of the analytical techniques has allowed a reduction in the complexity of the sample treatment and has increased the accuracy and precision of the analysis. One of the first multi-residue methods for organochlorine pesticides analysis in food was developed in 1963 using acetonitrile and petroleum ether [99]. To be able to analyse more polar pesticides than the organochlorine group, Luke et al. [100] validated a method based on acetone followed by dichloromethane and petroleum ether partitioning and clean-up with Florisil. An acetone based extraction method was also developed in 1983 by the Dutch Food and Consumer Products Safety Authority-Food Inspection Service [101] which was routinely applied for pesticide monitoring during more than 25 years. The Swedish National Food Administration developed an analytical method using ethyl acetate combined with a clean-up by gel permeation chromatography in 1989 [53]. Ethyl acetate is less polar (polarity index 4.4) than acetone (polarity index 5.1) and so polar pesticides partition less in ethyl acetate. To push the polar pesticides into the organic solvent large amounts of the anhydrous sodium sulphate (Na2SO4) are added to the water phase. In 2003, Anastassiades at al. [102] introduced a new strategy based on acetonitrile extraction followed by a clean-up using dispersive solid phase extraction (dSPE) with a primary and a secondary amine (PSA) and octadecylsilyl (C18). They termed this sample treatment procedure as QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe). This method became popular because of its minimal use of traditional analytical steps, solvent and glassware. This resulted in the publication of two reference methods: the first one published by the European Committee for Standardisation (CEN-15662) [10] which used acetonitrile with a citrate buffer during the extraction; the second one published by AOAC International as “Method 2007.01”, using acetonitrile with an acetate buffer during the extraction [12].
Many of the methods published for the analysis of pesticides in grapes are based on the QuEChERS methodology. Tables 2 and 3 show the increasing popularity of QuEChERS during the last decade. Out of a total of 55 published multi-residue methods between 2000 and 2014, 21 (or 38 %) are based on the QuEChERS methodology (taking both acetonitrile and ethyl acetate as possible extraction solvents in the QuEChERS methodology). This percentage increases in the case of multi-residue methods (Table 2) to 63 % when only the last 5 years (2009–2014) are taken into account (10 out of 16).
In general, the common procedure to analyse a large number of pesticide residues in grapes (Table 2) uses acetonitrile [33], [48], [52], [57], [61], [63], [65], [66] and [69] or ethyl acetate [49], [50], [51], [53], [54], [55], [58], [59], [60], [62], [64], [70] and [71] as organic solvents (Fig. 1). In the outline of the extraction performance with ethyl acetate an evolution is observed from a larger volume (40–200 mL) of ethyl acetate [49], [50], [51], [53], [55] and [76] to a reduction of the solvent volume to around 10 mL [54], [58], [59], [60], [62], [64], [68], [70], [71], [81] and [86] This decrease of volume allowed the elimination of the drying step for sample pre-concentration before sample injection into the LC or GC instrument. One of the shortcomings of the ethyl acetate extraction is the loss of basic pesticides in acidic crops like grapes. To overcome this problem NaHCO3 was added successfully by Pihlström et al. [53]. Three exceptions from this common procedure can be found in Table 2: an extraction based on acetone [56], a procedure using tetrafluoroethane-toluene and pressurised liquid solvent extraction [5] and a mixture of acetonitrile and methanol (90:10, v/v) [72]. A recent comparison of two QuEChERS methods (one citrate-buffered and one acetate-buffered) in different fruit matrices showed that the acetate-buffered method was more efficient and appropriate for grapes [103].
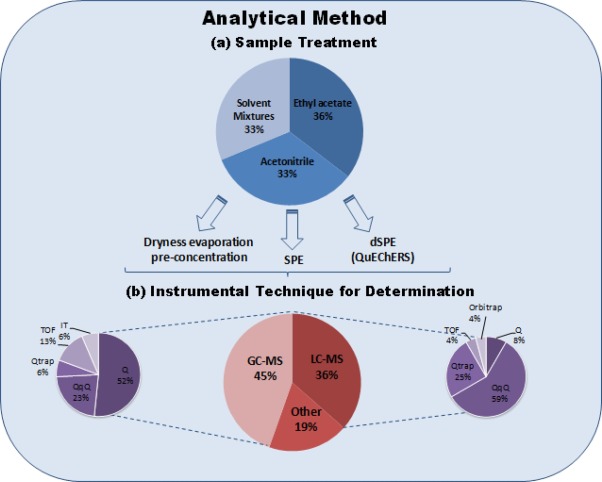
Fig 1. Scheme summarising the information of the analytical methods included in Table 2 and Table 3: (a) sample treatment; and, (b) instrumental techniques for analysis.
The extraction solvents used for the solid-liquid extraction in the case of specific methods for grapes and their by-products (Table 3) show a higher versatility. Apart from the use of acetonitrile [38], [74], [80], [89], [93] and [94] and ethyl acetate [75], [76], [81], [85], [86] and [98], other organic solvents have also been used: acetone [82], [84] and [88]; methanol [32], [79] and [90]; ethanol [95]; and, deionized water [83] and [87]. One of the reasons for this higher variability in extraction solvents may be that specific methods are developed and optimised for a small group of pesticides (often from the same chemical family and analysed by GC).
A study of the extraction solvent selection for 6 organophosphorus pesticides with a low molecular mass, very polar and/or thermolabile has been conducted in reference [104]. The solvents investigated were water, methanol, acetone (with and without partitioning in dichloromethane-petroleum ether) and ethyl acetate. Ethyl acetate was most favourable with respect to matrix effects, interferences in LC–MS/MS and extraction efficiency. After analysing all methods included in Table 2 and 3, the preferred solvents for pesticide residue analysis in grapes are ethyl acetate and acetonitrile, as shown in Fig. 1.
In a few of the published methods bases like sodium hydroxide [38] and [66], ammonium formate [89] or ammonium acetate [90] and [95] are added to the extraction solvent in order to neutralise the acid matrices.
The most common mixing and homogenising tools used in the extraction process are a probe blender or UltraTurrax [49], [55], [76], [77], [82], [84] and [96]. As alternative techniques, the application of the ultrasonic assisted extraction (UAE) [67], [79],[81] and [97], microwave assisted extraction (MAE) [88], or pressurised liquid extraction (PLE) [5] and [97] have been described. A comparison of 4 extraction approaches have been carried out for 8 pesticides (dichlofluanid, vincozolin, penconazole, captan, quinoxyfen, fluquinconazol, pyraclostrobin and boscalid) in grapes by Lagunas-Allué et al. [105]. MAE, solid–liquid extraction (SLE), QuEChERS and matrix solid-phase dispersion (MSPD) were compared in this study. Recoveries were in the range 78–100% (MAE), 66–102% (MSPD), 58–88% (SLE) and 68–96% (QuEChERS). The lowest LOQs were achieved with MAE and the highest with QuEChERS and SLE.
Clean-up of the extract
The preliminary extraction with organic solvents is mostly followed by a clean-up step. Different approaches are described below.
Partitioning with liquid–liquid extraction (LLE) is the most traditional strategy for clean-up. It is derived from the Luke method [100] and nowadays is not commonly used in multi-residue methods (Table 2). Just like in the Luke method LLE often follows an acetone extraction and uses a solvent mixture containing dichloromethane. Dichloromethane has been used alone or combined with e.g., light petroleum [56] or petroleum ether [39]. LLE has been more often applied in specific methods for grapes and by-products (Table 3) than in multi-residue methods (Table 2). This may be related to the fact that in specific methods the solvents can be more easily adapted to the specific pesticides to be analysed. Some methods are based on a mixture of acetone:dichloromethane [73],[82] and [84]. Additionally other solvent mixtures have been used such as ethyl acetate combined with hexane [75] and [81], dichloromethane [85] or acetone [92]; acetone combined with hexane [88]; and cyclohexane combined with dichloromethane [77]. In some cases [81], [82] and [84] salts like anhydrous sodium sulphate or sodium chloride are added in order to increase the separation between the liquid phases or increase the recovery of the pesticides.
Solid phase extraction (SPE) is widely accepted as an alternative clean-up method for LLE (Figure 1). Advantages are smaller volume of solvents and cleaner extracts. The typical SPE columns used for the clean-up of multiple types of pesticides in fresh fruits and vegetables (including grapes) were evaluated by Schenck et al. [106]. This study included reverse phase columns such as octadecylsilyl (C18), aminopropyl (-NH2) and primary-secondary amine (PSA), anion exchange columns such as trimetyl ammonium strong anion exchange (SAX) and adsorbents such as graphitized carbon black (GCB). This work concluded that the bonded normal phase SPE columns (-NH2 and PSA) were the most effective in removing the matrix co-extractants, especially fatty acids (hexadecanoic and octadecanoic acids), while the GCB sorbent removed pigments but did not remove noticeable chromatographic interferants. The C18 and SAX phases also removed relatively little of the co-eluting matrix co-extractants of the tested fruits and vegetables. Melo et al. [107] also compared in-house made polysiloxanes (aminopropyl-terminated poly(dimethylsiloxane) and poly(methyloctadecylsiloxane)) SPE columns with the commercial NH2 and C18. For the 6 pesticides checked in grapes in this paper, cartridges with amino-based material generated better results than the octadecyl sorbents, with the best performance for the 40% aminopropyl-loading SPE columns. SPE was only implemented in two of the multi-residue methods included in Table 2[52] and [72] and four of the specific methods for grapes and by-products in Table 3[32],[78], [81] and [90]. Different phases were applied in these studies. It is remarkable the large range of recoveries obtained for the different pesticides when using SPE (Table 2and Table 3) showing that care should be taken when selecting the SPE sorbent.
Nowadays, dSPE is mostly selected for the clean-up (Fig. 1). More than half of the multi-residue methods in Table 2 selected dSPE as a clean-up option. The dSPE methodology is based on SPE principles, but the solid phase (commonly PSA, C18 and/or GCB) is added directly to the extract without conditioning, and the clean-up is easily performed by shaking and centrifugation. This clean-up became very common with the implementation of the QuEChERS method in pesticide residue analysis. Its use is normally combined with the solvents acetonitrile [33], [38], [48], [57], [61], [65], [69], [80], [89], [93] and [94]and ethyl acetate [54], [58], [60], [63], [64], [68], [70], [71], [86] and [98]. The preferred adsorbent for this application is PSA that removes sugars and fatty acids, and which is included in all the dSPE strategies shown in Table 2 and Table 3. The use of magnesium sulphate is also standardized in dSPE, especially for GC applications to eliminate water from the organic solvent. Additionally, GCB is included in dSPE to remove pigments and sterols in samples, while C18 is used to remove non-polar interferences such as lipids. Different combinations of solid phases used for the dSPE in grape analysis have been described: (a) 25 mg/mL PSA is the most simple dSPE introduced by the group of K. Banerjee [54], [58], [60], [62], [64], [70] and [86]; (b) 12–40 mg/mL of PSA and 50–160 mg/mL magnesium sulphate [38], [48], [57], [61], [65], [66] and [93]; (c) 20–50 mg/mL PSA, 60–150 mg/mL magnesium sulphate and 10–175 mg/mL C18[69], [80] and [98]; and, (d) 25 mg/mL PSA and 7–25 mg/mL GCB [68] and [71]. Mol et al. [55] investigated the adsorption of pesticides with planar functionality on GCB during dSPE. Different ratios of toluene/GCB for the dSPE were evaluated for the recovery of 35 pesticides with a planar functionality (out of a total number of 341 pesticides in the multi-residue method), concluding that 20% of toluene was the most satisfactory approach.
A simultaneous extraction and clean-up, MSPD, has also been applied as an elegant alternative. It uses solid phases like C8[59] or C18[85]. The difference between MSPD and SPE is that MSPD can handle solid or viscous liquid samples directly, while SPE needs a previous solid-liquid extraction. In MSPD, the sample is homogeneously mixed with the solid phase and then placed in a column to proceed to the elution like in SPE. Reversed-phase materials such as C8 and C18 with a lipophilic character enable a good disruption of the matrix and a good adsorption of the compounds on the adsorbent. Ramos et al. [59] developed and validated an analytical method for 38 multiclass pesticides in different matrices including grapes, with the remarkable miniaturised C8-MSPD-based method involving a small amount of sample and solvent (i.e., 100 mg and 700 μL of ethyl acetate). In the work of Lagunas-Allué et al. [85] a C18-MSPD-based sample treatment for the analysis of 8 fungicides in grapes is described.
In order to reduce the use of organic solvents, other approaches have also been applied such as the stir bar sorptive extraction (SBSE) for the determination of 18 multiclass pesticides [79] and 7 strobilurin fungicides [95]; and, such as the solid phase microextraction (SPME) to determine 10 triazole fungicides in grapes [96]. These techniques are based on adsorption of organic analytes from liquid samples on to a stationary phase of polydimethylsiloxane (PDMS), which is a fused-silica fiber in the case of SPME and a magnetic stirring bar for SBSE. After the analytes are transferred to the polymer coating, they are thermally desorbed in the GC injector. The advantages of these two approaches are good analytical performance, simplicity, low cost and elimination of organic solvents. The disadvantages of those recent techniques are the relatively long equilibrium time and the possible carry-over. In the same line but with lower extraction time, a novel extraction technique called dispersive liquid–liquid microextraction (DLLME) has been applied for 8 multiclass pesticides in table grapes [83]. This approach is based on a ternary component solvent extraction system: extraction solvent, disperser solvent and aqueous samples containing the analyte of interest. The hollow fibre sorptive extraction (HFSE) is also considered as a simple treatment technique based on the partitioning of the analytes between sorbent and sample solution. HFSE SiO2 hollow fibre as extraction sorbent has been applied by Li et al. [87] obtaining suitable recoveries of 25 diverse pesticides.
Common instrumental techniques for analysis
Data in Tables 2 and 3 shows that two analytical strategies based on GC and LC are used for pesticide residue analysis in grape samples (Fig. 1). The first analytical approach for pesticides residue analysis used GC, 3 detectors designed for GC appear in the oldest methods in Table 3: electron capture detector (ECD), nitrogen and phosphorus detector (NPD), and flame photometric detector (FPD). These detectors presented high sensitivity and selectivity for particular pesticides of interest: the ECD seemed especially useful for halogenated compounds such as organochlorine pesticides [59], [47], [61], [65], [70] and [72]; the NPD [61], [65], [66] and [70] was a very sensitive detector for organophosphorous and nitrogenated pesticides; and the FPD [72] and [79] was a specific analyser for sulphur and phosphorous pesticides. This explains why these detectors are more often used in the specific methods (Table 3) and less in the multi-residue methods (Table 2) as the latter want to analyse all classes of pesticides at once. The original detectors used for LC based methods were the UV or diode array detector (DAD) [32], [47], [62], [63] and [71]. However, nowadays the use of MS [5], [33], [38], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [79], [80], [81], [82], [85], [86], [87], [88], [89], [90], [92], [93], [94], [96], [97] and [98] is preferred by most laboratories due to its higher selectivity and sensitivity for all the pesticides (Fig. 1). The current trend is the use of tandem MS (MS/MS) and high resolution MS (HRMS). The most common MS analyzers used in grape analysis are: single quadrupole (Q) [33], [52], [55], [57], [59], [61], [70], [73], [74], [75], [79], [80], [85], [87], [88], [89], [94] and [97], triple quadrupole (QqQ) [5], [38], [48], [53], [55], [56], [66], [67], [68], [71], [76], [82], [90], [92], [93], [97] and [98], ion trap (IT) [57], [77] and [81], hybrid quadrupole ion trap (QTrap) [48], [53], [62], [63] and [65], time of flight (TOF) [54], [60], [64], [72] and [96] and Orbitrap [69].
GC–MS based methods
The most conventional GC–MS detector for pesticide analysis in grapes is the single quadrupole. After injection of the sample, separation is typically done on a fused-silica capillary column (5% phenyl, 95% dimethylpolysiloxane; 30 m × 0.25 mm × 0.25 μm), followed by electron ionization (EI, 70 eV) using split/splitless injection [52], [57], [59],[61], [70], [73], [75], [77], [85], [87], [88] and [94]. Compared to the classical EI, chemical ionization (CI) is less commonly used for the analysis of pesticide residues in grapes[42] and [66]. EI and positive CI can be applied for nearly every analyte (even neutral analytes). In negative CI the analytes need the presence of an acidic group or an electronegative group (like halogen atoms) in order to stabilise in the negative charge. Therefore the negative CI mode provides better selectivity for most typical pesticides as they possess these electronegative groups. The group of Dong et al. [66] developed and validated an analytical method based on GC with negative CI for the analysis of 82 pesticides in cabbage and apple, which they applied on a grapes sample. Two pesticide residues were detected in the grapes sample at levels lower than the current European MRL [6]: pyrifenox-E at 0.27 μg/kg and pyridaben at 0.32 μg/kg.
When MS analyzer is used, the acquisition modes mainly selected are single ion monitoring (SIM) and full scan m/z 50–600. In order to achieve a valuable identification and quantification of the analyte at least 2–3 ions are selected in SIM mode.
As an alternative to the single quadrupole, the ion trap (IT) has also been applied, in which a scan acquisition mode allows the ion selection to be monitored post-acquisition. Nowadays, combinations of most MS analyzers are possible, allowing tandem-MS (QqQ) to be performed. The use of QqQ has been introduced in routine-analysis of pesticide residues in grapes [48], [53], [66], [68], [97] and [98]. This resulted in an improvement of the sensitivity and selectivity of the analytical methods. Tandem MS gives the possibility of measuring in selected reaction monitoring (SRM), which is a very selective acquisition mode. The potential matrix interferences are minimized or eliminated achieving lower limits of detection by reducing the chemical noise of the chromatogram. The QqQ proposed methods in grapes select in general at least 2 SRM transitions per analyte. However, due to the small m/z ratio of the pesticide and the use of EI as ionization source, the number and/or abundance of ions may be poor or make it difficult to obtain two suitable transitions, for example: mepanipyrim with the transitions 223 > 222, 222 > 220 and 222 > 118 [48]; binapacryl or pyrimethanil which SRM were 83 > 55 and 83 > 83, or 199 > 198 and 198 > 118, respectively [53]; acenaphthene with SRM 154 > 153 and 152 > 150 [56]. In other cases the m/z of the fragment is too low to be selective, as it is the case of the m/z 35 for the chlorine atom. The selection of m/z is very specific for chlorine but not good enough to discriminate between different chlorinated pesticides that can have very close retention times. Some of the examples are dicloran (206 > 35), quintozene (264.8 > 35), vinconzolin (241 > 35), tetrachlorvinphos (405.6 > 35), or beta-endosulfan (405.6 > 35) [66]. In this low selective SRM acquisition, the reliability of the identification and the quantification of the analyte may be compromised.
LC–MS based methods
Liquid chromatographic separation of pesticides has been performed by reversed phase (RP), due to the polarity of these analytes. The common stationary phases are based on C18[48], [51], [52], [53], [54], [55], [56], [57], [63], [65], [67], [69], [72], [74], [75], [90],[93] and [95]. In general the mobile phase for the analysis of pesticides with RP-LC consists of mixtures of water-methanol [32], [48], [49], [50], [51], [53], [54], [55], [56], [57],[63], [65], [67], [72] and [90], and water-acetonitrile [52], [69], [74], [75], [83], [93] and [95]. In order to improve the ionization capacity, the use of different additives to the mobile phases have been described, such as ammonium acetate [49], [50], [69] and [82] or ammonium formate [46], [48], [53], [54], [55], [56], [63], [65], [67] and [76] (concentration level of 5–10 mM), and formic acid [51], [57], [72], [90] and [93] (normally at a concentration level of 0.1%). The injection volumes are usually between 5 and 25 μL for a flow rate of 200–300 μL/min. One technological revolution in LC has been the implementation of ultra-high pressure liquid chromatography (UHPLC). In UHPLC the particle size of the solid phase is reduced from 5 to 3 μm [46], [48], [49], [50], [51], [52],[53], [54], [55], [56], [57], [63], [65], [67], [74], [76], [82], [83], [90] and [95] to sub-2 μm [5],[69] and [72] resulting in enhanced resolution in a shorter runtime. For instance, the group of Sivaperumal et al. [72] achieved the separation of 60 pesticides in less than 5 min with average peak widths of 10 s.
Ionization in LC–MS is usually performed by atmospheric pressure ionization (API) sources. API has the capacity of obtaining abundant intact protonated molecules. The most used API source is electrospray (ESI) in positive mode. Only one paper [74]selected atmospheric pressure chemical ionization (APCI) for the analysis of 12 botanical insecticides in grapes. APCI could be a very suitable alternative to obtain abundant ionization of analytes without acidic or basic centre, as was the case for these 12 botanical insecticides.
Due to the characteristic soft ionization source API, LC is coupled directly to tandem MS analyzers, such as the QqQ or Qtrap (Fig. 1). This explains why the QqQ [46], [49], [50],[51], [56], [76] and [82] and QTrap [48], [52], [53], [54], [55], [57], [63], [65], [90] and [93]are the most common MS analyzers in LC-based methods for pesticide residues analysis. Both MS/MS analyzers are used for quantification and confirmation purposes. Typically 2 or 3 SRM transitions are selected for target analysis of pesticide residues: one for quantification and an additional one for confirmation purposes. The use of the QTrap presents the advantage of very sensitive scan acquisition in the second analyzer.
High resolution MS-based methods
Finally, the use of high resolution MS (HRMS) instruments has been introduced for quantitative pesticide residues analysis in grapes. The HRMS analyzers used for pesticide residue analysis in grapes are the QTOF [58], [60], [64], [72] and [96] and QOrbitrap [69]. One of the main attributes of the HRMS analyzers is their accurate mass measurements, increasing the reliability of the analyte detection by providing extra selectivity by elemental composition of parent and fragment ion spectra.
Only few papers have described the use of HRMS for quantitative purposes in grape pesticide residue analysis. A quantitative method for 10 triazole pesticides in grapes by GC-TOF was validated by Souza-Silva et al. [96]. One m/z ion was selected for quantification and specific software for the deconvolution was applied in order to obtain pure mass spectra used for identification in case of co-elutions. Dasgupta et al. [64]validated a method for 135 pesticides based on GC-TOF by selecting a single diagnosticm/z ion for each analyte. Two-dimensional gas chromatography (GC × GC) coupled to TOF has also been applied for quantitative purposes by the group of Banerjee[58] and [60], leading to a method for the analysis of 160 pesticides within 38 min [60]. Sivaperumal et al. [72] developed and validated a method based on UHPLC-TOF for 60 pesticides in different commodities including grapes. In this work the accurate mass measurements were discussed for qualitative purposes, and the mass measurements were reported with an accuracy level <2.3 ppm. Although the use of TOF analyzers is an attractive tool for accurate mass measurements, it is not so much exploited for quantitative purposes [108]. The papers presented [58], [60], [64], [72] and [96] have selected a single diagnostic ion for quantification. However, the extraction of the ion from the total ion chromatogram is not specified by using a mass accuracy threshold or range.
The QOrbitrap [69] has also been applied in the determination of 166 pesticides in different fruit samples. In this approach an acquisition is done either in full MS-SIM or in full MS/data dependant MS2, both in positive mode. In case of target compounds detected inside the ion abundance threshold and mass error (10 ppm error mass window), the product-ion spectra were obtained by selection within a window 4.0 m/z in the quadrupole to be sent to the HCD collision cell of the QOrbitrap mass spectrometer. The accurate mass measurement was established at <5 ppm for identity confirmation of the analyte; as example carbendazim was identified within 0.9 and 1.1 ppm for the precursor ion and the product ion.
Although the new generation of HRMS analyzers can be applied for quantitative analysis, the typical purpose of these analyzers is much more to focus on the development of screening methods for post-acquisition non-target analysis in food [108], [109] and [110]. The most common instruments for quantitative target analysis in multi-residue methods remain the QqQ and QTrap, due to the high sensitivity and selectivity in target multi-residue methods.
Quantitative analysis and matrix effect
The use of chromatographic techniques coupled to MS can often produce very reliable methods for the determination of pesticides at trace level in grapes. However, matrix interferences can compete with the analyte of interest and compromise the selectivity and specificity of the method. The effect of these matrix interferences can be compensated for after studying them and applying different approaches. The best choice is the use of stable isotopically labeled standards for each analyte. However, this option can be very expensive, and therefore other alternatives have been proposed in the papers reviewed.
The most universally adopted strategy is the use of matrix-matched calibration by preparing the standard solution in a blank grape extract. In all multi-residue methods included in Table 2, matrix-matched calibration has been used to correct the effects of the matrix interferences. For instance, Taylor et al. [49], Hiemstra et al. [56] or Mol et al. [55]decided to check the matrix effect in each run by comparing the matrix-matched calibration with the standard calibration. Like this they could evaluate the matrix effect over a large number of analyses and study its influence on the accuracy of quantification.
In methods using matrix-matched calibration sometimes an internal standard such as triphenylphosphate [48] and [57] or heptachlor-epoxide [61] was introduced to correct the errors derived during the sample treatment and/or instrumental analysis. In a few methods, isotopically labelled pesticide standards were added during the analysis [33],[65], [67] and [69]. For example, in the multi-residue method of Zhang et al. [65] six deuterium labeled internal standards were introduced (dimethoate-d6, dichlorvos-d6, diuron-d6, linuron-d6, dichlorvos-d6 and malathion-d6). However, those standards were used to check the quality of the analysis and to estimate the matrix effect, but not for quantitative purposes. Only in two analytical methods [67] and [69] isotopic dilution mass spectrometry was used for quantitative purposes.
Most of the single-residue methods for grapes included in Table 3 also used matrix-matched calibration [38], [76], [77], [78], [80], [83], [84], [85], [86], [88], [89], [90], [91],[92], [93], [94], [96] and [98]. Only few of them used a combined strategy with matrix-matched calibration and an internal standard, such as triphenylphosphate [78] and [80], tris-(1,3-dichloro-2-propyl) phosphate [89], diniconazole [91], tetradifon [85] and [88], tebuconazole-d6 [96], and parathion ethyl-d10 [98].
Limitations of multi-residue methods
The most common and efficient way to carry out pesticide residue analysis for hundreds of different compounds is the use of multi-residue methods able to measure in the MRL range from 0.01 to 10 mg/kg. Unfortunately, these multi-residue methods cannot measure all pesticides with the required accuracy in one single run. The high diversity in chemical composition of these hundreds of pesticides compromises the use of a single strategy for their simultaneous analysis. This explains why in some cases it is still necessary to develop single-residue methods for the analysis of one pesticide or a few pesticides from the same chemical family. Examples are pesticides with a high polarity or with an ionic character. Another problem may be the low stability of specific pesticides during sample extraction.
In the case of compounds with a high polarity or ionic compounds, new approaches based on LC have been proposed. They can be divided in three strategies: (i) the polarity of the analytes is reduced by derivatization of the analytes or by addition of an ion-pairing substance to the mobile phase before analysis by RP-LC. This decreased polarity leads to an increased retention and more adequate peak shape [111]. (ii) Use of hydrophilic interaction liquid chromatography (HILIC) with carbon or ion exchange phases instead of reversed phases. Also this leads to an increased retention of the analytes [112] and [113]. (iii) Elimination of the separation technique and use of direct flow injection (FI) to MS/MS[114].
For instance, a specific single method for the analysis of ethephon using UHPLC-QqQ has been described by Hanot et al. [115]. Ethephon is a stable molecule in aqueous solutions below pH 4 and is decomposed in ethylene and dihydrogen phosphate under alkali and high temperature conditions. Due to its high polarity it cannot be included in a multi-residue method. Its chromatographic separation was obtained by using a HILIC column with addition of ammonium hydroxide to the aqueous mobile phase. Anastassiades et al. [113] studied ion chromatography using anion exchange for the analysis of highly polar pesticides in food (including grapes). This method was applied for ethephon, 2-hydroxyethephon (HEPA, ethephon metabolite), glyphosate, aminomethylphosphonic acid (AMPA, glyphosate metabolite), N-acetyl AMPA, glufosinate, N-acetyl-glufosinate, 3-methylphosphinico propionic acid (MPPA, glufosinate metabolite), fosetyl-Al and phosphonic acid.
The dithiocarbamate fungicide residues represent one of the most complex groups to be determined due to their low stability in vegetable matrices and low solubility in water or polar organic solvents. Therefore, it is difficult to include these pesticides in the scope of a multi-residue method. Three methods for the analysis of dithiocarbamate fungicides in grapes have been described [116]. They all use an extraction with alkaline buffer followed by HILIC chromatography and LC–MS/MS detection.
Future perspectives
One tendency seen in the sample treatment for GC applications is the use of alternative solid phases, such as the multi-walled carbon nanotubes (MWCNTs) [78] and [94]. This material has been effectively used in SPE for grape juice [78], or in dSPE replacing PSA in the QuEChERS workflow [94]. Another interesting novelty for GC–MS applications is the introduction of the atmospheric pressure chemical ionization (APCI) source, which gives a more soft ionization and more selective fragmentation. The integration of the APCI at GC-QqQ analyzers has demonstrated a strong potential to improve the abundance of the product ions leading to increased sensitivity and selectivity. For instance, Portoles at al. [117] have presented a work to evaluate the performance of GC-APCI-QTOF for screening of 132 pesticide residues in several vegetable matrices including grapes. In order to test the screening capacity of the method, blank samples were spiked with the 132 pesticides at 0.01 mg/kg. Detection was based on the extracted ion chromatogram of one diagnostic ion (exact mass ± 75 ppm and time window ± 0.2 min). With this approach 89% of the pesticides in 20 samples were found.
In LC–MS analysis a further minimisation of the sample treatment is obtained with single solvent extraction and/or dilution and direct injection in the LC system [118]. An even further simplification without the use of an LC system can be achieved by the use of flow injection FI-MS [119]. One of the main drawbacks in the application of this strategy is the matrix effect, which endangers the traceability of the quantification. In the last two years, the use of high-throughput planar solid phase extraction (HTpSPE) was established as a new clean-up concept, resulting in matrix-free extracts with almost no interferences. HTpSPE combined a fully automated sample application and plate development with the thin layer chromatography–MS interface as the essential tools of the method. Oellig et al.[120] applied HTpSPE clean-up combined with FI-TOF-MS in a grape sample for screening analysis, omitting the LC separation step. In the recovery experiments for 7 pesticides (azoxystrobin, chlorpyrifos, fenarimol, penconazole, pirimicarb, mepanipyrim and acetamiprid) the use of HTpSPE for clean-up demonstrated a very efficient option in order to eliminate the matrix effect, obtaining near-100% recovery values.
Recently, ambient desorption/ionization (ADI) appears to be a powerful method that reduces the need for sample preparation and separation techniques like GC and LC. The ADI has been applied for pesticide residue analysis in grapes for qualitative and quantitative aims. Direct analysis in real time (DART) [121] and micro-fabricated glow discharge plasma (MFGDP) [122] have proven to be useful in screening purposes, while a low-temperature plasma (LTP) probe [123] has shown to be effective in quantitative analysis as well. In the work of Edison et al. [121] 132 pesticides were simply swabbed from the grapes surface, and then detected by DART-Orbitrap. Out of the 132 analytes, 86% of them were qualitatively detected at 10 ng/g concentration level. The great potential of ADI sources is the capability of providing a mapping of the pesticide distribution in the fruit [109]. However, the use of ADI for quantitative purpose could be still compromised because of the limited precision compared to the classical LC-ESI–MS[123].
The use of HRMS analyzers for quantitative purposes will most probably increase in the future. The further development of these new technologies and related software will increase the sensitivity of these detectors as well as their user-friendliness. This will allow screening and quantification with the same instrument and with the required sensitivity. Because of the increased selectivity of this HRMS acquisition, sample treatment may further be simplified leading to cost-effective analysis detecting a maximum number of pesticides with acceptable accuracy.
Conclusions
The revision of the analytical methods for grapes published during the last 10 years shows that there are a large number of strategies for the analysis of the wide range of pesticide residues which may be present in grapes. The most commonly used solvents for extraction are ethyl acetate and acetonitrile. This extraction is often followed by a SPE or dSPE sample clean-up. The QuEChERS methodology is the most common sample preparation technique, used in about 38% of the methods for grape analysis studied in this review. The instruments of choice for the analytical separation are both LC and GC. LC and GC are frequently coupled to a tandem MS/MS as QqQ for identification and quantification.
In this last 10 years, a number of improvements in the analytical methodologies for the analysis of pesticide residues in grapes have been achieved. Some of the main ones are: (a) the reduction of sample size and the quantity of organic solvents or other reagents in order to miniaturize the extraction process; (b) the automation of the sample preparation which resulted in a reduction of the errors in the manipulations and which improved the reproducibility and repeatability of the analytical methods; (c) better separation techniques leading to an increased resolution and reduced separation time; (d) development of screening methodologies with quantitative and confirmative capacities.
There is no universally accepted analytical method for pesticide residues analysis in grapes today. The large number of pesticides belonging to different chemical classes and the fact that these analytical methodologies need to be applicable to many other fruit and vegetable matrices make this wish hard to accomplish. Therefore, there is still a need for single-residue methods for the analysis of a few pesticides in grapes and its by-products. One of the reasons for this need is the fact that the novel pesticides are more polar as these have less impact on the environment. The use and occurrence of this type of pesticides will probably increase in the future.
References
[1]
International Organisation of Vine and Wine, World vitiviniculture situation 2015, (2015). http://www.oiv.int/oiv/info/enpublicationsstatistiques.
[2]
Usda
National nutrient database for standard reference, United States
Dept. Agric. (2012), p. 66 http://ndb.nal.usda.gov/ndb/foods/list
[3]
R. Flamini
Mass Spectrometry in Grape and Wine Chemistry. Part I: Polyphenols
John Wiley & Sons Inc. (2003) http://dx.doi.org/10.1002/mas.10052
[4]
P. Caboni, P. Cabras
Advances in Food and Nutrition Research
Elsevier Inc. (2010)
[5]
G.T. Bakırcı, Y. ßar, H. ßıl
Fast and simple extraction of pesticide residues in selected fruits and vegetables using tetrafluoroethane and toluene followed by ultrahigh-performance liquid chromatography/tandem mass spectrometry
Food Chem., 135 (2012), pp. 1901–1913 http://dx.doi.org/10.1016/j.foodchem.2012.06.051
[6]
Commission Regulation (EC) 396/2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin, J. Eur. Union. L70/1 (2005).
[7]
EURL-FV, Dutch mini-Luke (NL-) extraction method followed by LC and GC–MS/MS for multiresidue analysis of pesticides in fruits and vegetables, (2014) M12. http://www.eurl-pesticides.eu/userfiles/file/NL-miniLuke-extraction-method.pdf.
[8]
A. Lozano, B. Kiedrowska, J. Scholten, M. de Kroon, A. de Kok, A.R. Fernández-Alba
Miniaturisation and optimisation of the Dutch mini-Luke extraction method for implementation in the routine multi-residue analysis of pesticides in fruits and vegetables
Food Chem., 192 (2016), pp. 8–681 http://dx.doi.org/10.1016/j.foodchem.2015.07.065
[9]
European Committee for Standardization, Foods of plant origin- Multi-residue methods for the gas chromatographic determination of pesticide residues, EN 12393-1 (2008).
[10]
European Committee for Standardization, Foods of plant origin- Determination of pesticide residues using GC–MS and/or LC–MS/MS following acetonitrile extraction/partitioning and clean-up by dispersive SPE-QuEChERS-method, EN 15662 (2008).
[11]
European Committee for Standardization, Foods of plant origin- Determination of pesticide residues using LC–MS/MS following methanol extraction and clean-up using diatomaceous earth, EN 15637 (2008).
[12]
AOAC Official Method, Pesticide Residues in Food by Acetonitrile extraction and Partitioning with Magnesium Sulfate GC–MS and LC–MS/MS, 2007.01 (2007).
[13]
SANCO/12571/2013, Guidance document on analytical quality control and validation procedures for pesticide residues analysis in food and feed, (2014).
[14]
I.O. for Standardization
ISO/IEC 17025 General requirements for the competence of testing and calibration laboratories
Int. Stand., 3 (2005), pp. 1–36
[15]
P. Medina-Pastor, C. Rodríguez-Torreblanca, A. Andersson, A.R. Fernández-Alba
European Commission proficiency tests for pesticide residues in fruits and vegetables
Trends Anal. Chem., 29 (2010), pp. 70–83 http://dx.doi.org/10.1016/j.trac.2009.11.001
[16]
P. Dehouck, S. Grimalt, M. Dabrio, F. Cordeiro, et al.
Proficiency test on the determination of pesticide residues in grapes with multi-residue methods
J. Chromatogr. A, 1395 (2015), pp. 143–151 http://dx.doi.org/10.1016/j.chroma.2015.03.076
[17]
Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides, Off. J. Eur. Union. L 309/71 (2009).
[18]
European Commission, EU. Pesticides database, Regul. No 1107/2009. (2009). http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=homepage&language=EN.
[19]
W.J. Bentley
The integrated control concept and its relevance to current integrated pest management in California fresh market grapes
Pest. Manage. Sci., 65 (2009), pp. 1298–1304 http://dx.doi.org/10.1002/ps.1840
[20]
H.B. Česnik, A. Gregorčič, F. Čuš
Pesticide residues in grapes from vineyards included in integrated pest management in Slovenia
Food Add Contam., 43 (2008), pp. 8–443 http://dx.doi.org/10.1080/02652030701558490
[21]
C. Turgut, H. Ornek, T.J. Cutright
Determination of pesticide residues in Turkey’s table grapes: the effect of integrated pest management, organic farming, and conventional farming
Environ. Monit. Assess., 173 (2011), pp. 315–323 http://dx.doi.org/10.1007/s10661-010-1389-4
[22]
G.T. Bakırcı, D. Bengü, Y. Acay, F. Bakırcı, S. Ötles
Pesticide residues in fruits and vegetables from the Aegean region
Turkey, Food Chem., 160 (2014), pp. 9–392 http://dx.doi.org/10.1016/j.foodchem.2014.02.051
[23]
F.I. Eissa, A.A. Helalia, M.A. Khorshed, M.A. El-Sisi
Monitoring of multi-class pesticide residues in green grape and their potential risk for Egyptian consumer
Nat. Sci., 11 (2013), pp. 110–115 http://www.sciencepub.net/nature
[24]
M.E. Poulsen, H.K. Hansen, J.J. Sloth, H.B. Christensen, J.H. Andersen
Survey of pesticide residues in table grapes: Determination of processing factors, intake and risk assessment
Food Addit. Contam., 88 (2007), pp. 6–895 http://dx.doi.org/10.1080/02652030701245320
[25]
K. Hjorth, K. Johansen, B. Holen, A. Andersson, H.B. Christensen, K. Siivinen, et al.
Pesticide residues in fruits and vegetables from South America—a Nordic project
Food Control, 170 (2011), pp. 1–1706 http://dx.doi.org/10.1016/j.foodcont.2010.05.017
[26]
E. Pose-Juan, M.J. Sánchez-Martín, M.S. Andrades, M.S. Rodríguez-Cruz, E. Herrero-Hernández
Pesticide residues in vineyard soils from Spain: spatial and temporal distributions
Sci. Total Environ., 514 (2015), pp. 351–358 http://dx.doi.org/10.1016/j.scitotenv.2015.01.076
[27]
V.T. Gajbhiye, S. Gupta, I. Mukherjee, S.B. Singh, N. Singh, P. Dureja, et al.
Persistence of azoxystrobin in/on grapes and soil in different grapes growing areas of India
Bull. Environ. Contam. Toxicol., 86 (2011), pp. 90–94 http://dx.doi.org/10.1007/s00128-010-0170-2
[28]
S. Mohapatra, A.K. Ahuja, M. Deepa, G.K. Jagdish, N. Rashmi, S. Kumar, et al.
Persistence and dissipation of fluopicolide in/on grape berries and soil under semi arid tropical climatic conditions of India
Bull. Environ. Contam. Toxicol., 86 (2011), pp. 238–241 http://dx.doi.org/10.1007/s00128-011-0193-3
[29]
C. Kundu, A. Goon, A. Bhattacharyya
Persistence behaviour of fungicide tebuconazole in a viticulture application
Bull. Environ. Contam. Toxicol., 92 (2014), pp. 415–419 http://dx.doi.org/10.1007/s00128-014-1223-8
[30]
R. Sabale, T.P.A. Shabeer, S.C. Utture, K. Banerjee, M.R. Jadhav, D.P. Oulkar, et al.
Dissipation kinetics, safety evaluation, and assessment of pre-harvest interval (PHI) and processing factor for kresoxim methyl residues in grape
Environ. Monit. Assess., 186 (2014), pp. 2369–2374 http://dx.doi.org/10.1007/s10661-013-3544-1
[31]
M. Fujita, T. Yajima, K. Iijima, K. Sato
Comparison of the variability in the levels of pesticide residue observed in Japanese cabbage and grape units
J. Agric. Food Chem., 60 (2012), pp. 1516–1521 http://dx.doi.org/10.1021/jf2040059
[32]
M.J. Teixeira, A. Aguiar, C.M. Afonso, A. Alves, M.M.S. Bastos
Comparison of pesticides levels in grape skin and in the whole grape by a new liquid chromatographic multiresidue methodology
Anal. Chim. Acta, 513 (2004), pp. 333–340 http://dx.doi.org/10.1016/j.aca.2003.11.077
[33]
X.-M. Xu, S. Yu, R. Li, J. Fan, S.-H. Chen, H.-T. Shen, et al.
Distribution and migration study of pesticides between peel and pulp in grape by online gel permeation chromatography–gas chromatography/mass spectrometry
Food Chem., 16 (2012), pp. 1–169 http://dx.doi.org/10.1016/j.foodchem.2012.04.052
[34]
L. Lagunas-Allué, J. Sanz-Asensio, M.T. Martínez-Soria
Mobility and distribution of eight fungicides in surface, skin and pulp in grapes. An application to pyraclostrobin and boscalid
Food Control, 51 (2015), pp. 85–93 http://dx.doi.org/10.1016/j.foodcont.2014.10.028
[35]
K. Banerjee, A.K. Upadhyay, P.G. Adsule, S.H. Patil, D.P. Oulkar, D.R. Jadhav
Rate of degradation of λ-cyhalothrin and methomyl in grapes (Vitis vinifera L.)
Food Addit. Contam., 23 (2006), pp. 994–999 http://dx.doi.org/10.1080/02652030600838613
[36]
F.M. Malhat, H.a. Mahmoud
Dissipation and residues of mandipropamid in grape using QuEChERS methodology and HPLC-DAD
ISRN Anal. Chem., 2012 (2012), pp. 1–5 http://dx.doi.org/10.5402/2012/267596
[37]
P. Cabras, A. Angioni, Pesticide Residues in Grapes, Wine, and Their Processing Products, APRIL. 48 (2000) 10.1021/jf990727a.
[38]
T. Pazzirota, L. Martin, M. Mezcua, C. Ferrer, A.R. Fernandez-Alba
Processing factor for a selected group of pesticides in a wine-making process: distribution of pesticides during grape processing
Food Addit. Contam. Part A. Chem. Anal. Control Expo. Risk Assess., 30 (2013), pp. 1752–1760 http://dx.doi.org/10.1080/19440049.2013.815806
[39]
F. Čuš, H.B. Česnik, Š.V. Bolta, A. Gregorčič
Pesticide residues in grapes and during vinification process
Food Control, 21 (2010), pp. 2–1518 http://dx.doi.org/10.1016/j.foodcont.2010.04.024
[40]
R.M. González-Rodríguez, B. Cancho-Grande, J. Simal-Gándara
Decay of fungicide residues during vinification of white grapes harvested after the application of some new active substances against downy mildew
Food Chem, 125 (2011), pp. 549–560 http://dx.doi.org/10.1016/j.foodchem.2010.09.047
[41]
C. Kundu, A. Goon, A. Bhattacharyya
Persistence behaviour of fungicide mixture (benalaxyl-M 4% + mancozeb 65%) WP in grapes
Bull. Environ. Contam. Toxicol., 89 (2012), pp. 1253–1257 http://dx.doi.org/10.1007/s00128-012-0847-9
[42]
A. Angioni, F. Dedola, V.L. Garau, M. Schirra, P. Caboni
Fate of iprovalicarb, indoxacarb, and boscalid residues in grapes and wine by GC-ITMS analysis
J. Agric. Food Chem., 59 (2011), pp. 6806–6812 http://dx.doi.org/10.1021/jf2011672
[43]
P. Edder, D. Ortelli, O. Viret, E. Cognard, A. De Montmollin, O. Zali
Control strategies against grey mould (Botrytis cinerea Pers.: Fr) and corresponding fungicide residues in grapes and wines
Food Addit. Contam. Part A. Chem. Anal. Control Expo. Risk Assess., 26 (2009), pp. 719–725 http://dx.doi.org/10.1080/02652030802668578
[44]
C. Liu, K. Wan, J. Huang, Y. Wang, F. Wang
Behavior of mixed formulation of metalaxyl and dimethomorph in grape and soil under field conditions
Ecotoxicol. Environ. Saf., 84 (2012), pp. 112–116 http://dx.doi.org/10.1016/j.ecoenv.2012.06.030
[45]
D.T. Likas, N.G. Tsiropoulos
Fate of three insect growth regulators (IGR) insecticides (flufenoxuron, lufenuron and tebufenozide) in grapes following field application and through the wine-making process
Food Addit. Contam., 28 (2011), pp. 9–197 http://dx.doi.org/10.1080/19440049.2010.542184
[46]
N.G. Tsiropoulos, G.E. Miliadis, D.T. Likas, K. Liapis
Residues of spiroxamine in grapes following field application and their fate from vine to wine
J. Agric. Food Chem., 53 (2005), pp. 10091–10096 http://dx.doi.org/10.1021/jf052162q
[47]
R.M. González-Rodríguez, B. Cancho-Grande, A. Torrado-Agrasar, J. Simal-Gándara, J. Mazaira-Pérez
Evolution of tebuconazole residues through the winemaking process of Mencía grapes
Food Chem, 117 (2009), pp. 529–537 http://dx.doi.org/10.1016/j.foodchem.2009.04.030
[48]
P. Payá, M. Anastassiades, D. Mack, I. Sigalova, B. Tasdelen, J. Oliva, et al.
Analysis of pesticide residues using the quick easy cheap effective rugged and safe (QuEChERS) pesticide multiresidue method in combination with gas and liquid chromatography and tandem mass spectrometric detection
Anal. Bioanal. Chem., 389 (2007), pp. 1697–1714 http://dx.doi.org/10.1007/s00216-007-1610-7
[49]
M.J. Taylor, K. Hunter, K.B. Hunter, D. Lindsay, S. Le Bouhellec
Multi-residue method for rapid screening and confirmation of pesticides in crude extracts of fruits and vegetables using isocratic liquid chromatography with electrospray tandem mass spectrometry
J. Chromatogr. A., 982 (2002), pp. 225–236 http://dx.doi.org/10.1016/S0021-9673(02) 01610-2
[50]
C. Jansson, T. Pihlström, B.G. Österdahl, K.E. Markides
A new multi-residue method for analysis of pesticide residues in fruit and vegetables using liquid chromatography with tandem mass spectrometric detection
J. Chromatogr. A, 1023 (2004), pp. 93–104 http://dx.doi.org/10.1016/j.chroma.2003.10.019
[51]
D. Ortelli, P. Edder, C. Corvi
Multiresidue analysis of 74 pesticides in fruits and vegetables by liquid chromatography-electrospray-tandem mass spectrometry
Anal. Chim. Acta, 520 (2004), pp. 33–45 http://dx.doi.org/10.1016/j.aca.2004.03.037
[52]
C.-L. Fan, Y.-M. Liu, Y.-Z. Cao, J.-J. Zhang, X.-M. Li, Z.-Y. Li, et al., Determination of Residues of 446Pesticides in Fruits and Vegetables by Three-Cartridge Solid-Phase Extraction-Gas Chromatography-Mass Spectrometry and Liquid Chromatography-Tandem Mass Spectrometry, 89 (2006) 740–771. http://www.atypon-link.com/AOAC/doi/abs/10.5555/jaoi.89.3.740.
[53]
T. Pihlström, G. Blomkvist, P. Friman, U. Pagard, B.-G. Osterdahl
Analysis of pesticide residues in fruit and vegetables with ethyl acetate extraction using gas and liquid chromatography with tandem mass spectrometric detection
Anal. Bioanal. Chem., 389 (2007), pp. 1773–1789 http://dx.doi.org/10.1007/s00216-007-1425-6
[54]
K. Banerjee, D.P. Oulkar, S. Dasgupta, S.H.S.B. Patil, R. Savant, et al.
Validation and uncertainty analysis of a multi-residue method for pesticides in grapes using ethyl acetate extraction and liquid chromatography–tandem mass spectrometry
J. Chromatogr. A, 1173 (2007), pp. 98–109 http://dx.doi.org/10.1016/j.chroma.2007.10.013
[55]
H.G.J. Mol, A. Rooseboom, R. Van Dam, M. Roding, K. Arondeus, S. Sunarto., Modification and re-validation of the ethyl acetate-based multi-residue method for pesticides in produce, 389 (2007) 1715–1754. 10.1007/s00216-007-1357-1
[56]
M. Hiemstra, A. De Kok
Comprehensive multi-residue method for the target analysis of pesticides in crops using liquid chromatography–tandem mass spectrometry
J. Chromatogr. A, 1154 (2007), pp. 3–25 http://dx.doi.org/10.1016/j.chroma.2007.03.123
[57]
C. Lesueur, P. Knittl, M. Gartner, A. Mentler, M. Fuerhacker
Analysis of 140 pesticides from conventional farming foodstuff samples after extraction with the modified QuECheRS method
Food Control, 19 (2008), pp. 906–914 http://dx.doi.org/10.1016/j.foodcont.2007.09.002
[58]
K. Banerjee, S.H. Patil, S. Dasgupta, D.P. Oulkar, S.B. Patil, R. Savant, et al.
Optimization of separation and detection conditions for the multiresidue analysis of pesticides in grapes by comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry
J. Chromatogr. A, 1190 (2008), pp. 350–357 http://dx.doi.org/10.1016/j.chroma.2008.03.017
[59]
J.J. Ramos, L. González, L. Ramos
Comparison of gas chromatography-based approaches after fast miniaturised sample preparation for the monitoring of selected pesticide classes in fruits
J. Chromatogr. A, 1216 (2009), pp. 7307–7313 http://dx.doi.org/10.1016/j.chroma.2009.05.013
[60]
S. Dasgupta, K. Banerjee, S.H. Patil, M. Ghaste, K.N. Dhumal, P.G. Adsule
Optimization of two-dimensional gas chromatography time-of-flight mass spectrometry for separation and estimation of the residue of 160 pesticides and 25 persistent organic pollutants in grape and wine
J. Chromatogr. A, 1217 (2010), pp. 3881–3889
[61]
Y.-J. Lian, G.-F. Pang, H.-R. Shu, C.-L. Fan, Y.-M. Liu, J. Feng, et al.
Simultaneous determination of 346 multiresidue pesticides in grapes by PSA-MSPD and GC–MS-SIM
J. Agric. Food Chem., 58 (2010), pp. 9428–9453 http://dx.doi.org/10.1021/jf1019592
[62]
R.H. Savant, K. Banerjee, S.C. Utture, S.H. Patil, S. Dasgupta, M.S. Ghaste, et al.
Multiresidue analysis of 50 pesticides in grape, pomegranate, and mango by gas chromatography-ion trap mass spectrometry
J. Agric. Food Chem., 58 (2010), pp. 1447–1454 http://dx.doi.org/10.1021/jf903398f
[63]
A. El-Moneim, M.R. Afify, M.A. Mohamed, H.A. El-Gammal, E.R. Attallah
Multiresidue method of analysis for determination of 150 pesticides in grapes using quick and easy method (QuEChERS) and LC–MS/MS determination
J. Food Agric. Environ., 88 (2005), pp. 602–606
[64]
S. Dasgupta, K. Banerjee, K.N. Dhumal, P.G. Adsule
Optimization of detection conditions and single-laboratory validation of a multiresidue method for the determination of 135 pesticides and 25 organic pollutants in grapes and wine by gas chromatography time-of-flight mass spectrometry
J. AOAC Int., 94 (2011), pp. 273–285 http://www.ncbi.nlm.nih.gov/pubmed/21391504
[65]
K. Zhang, J.W. Wong, O.P. Yang, K. Tech, A.L. Dibenedetto, N.S. Lee, et al.
Multiresidue pesticide analysis of agricultural commodities using acetonitrile salt-out extraction, dispersive solid-phase sample clean-up, and high-performance liquid chromatography à tandem mass spectrometry
J. Agric. Food Chem. 59, 763 (2011), pp. 6–7646 http://dx.doi.org/10.1021/jf2010723
[66]
J. Dong, Y.-X. Pan, J.-X. Lv, J. Sun, X.-M. Gong, et al.
Multiresidue method for the determination of pesticides in fruits and vegetables using gas chromatography-negative chemical ionization-triple quadrupole tandem mass spectrometry
J. Agric. Food. Chem., 59 (2011), pp. 109–119 http://dx.doi.org/10.1007/s10337-011-2055-x
[67]
L. Hollosi, K. Mittendorf, H.Z. Senyuva
Coupled turbulent flow chromatography: LC–MS/MS method for the analysis of pesticide residues in grapes, baby food and wheat flour matrices
Chromatographia, 75 (2012), pp. 7–1393 http://dx.doi.org/10.1007/s10337-012-2329-y
[68]
P. Banerjee, K. Utture, S. Dasgupta, S. Kandaswamy, C. Pradhan, S. Kulkarni, S. Adsule
Multiresidue determination of 375 organic contaminants including pesticides, polychlorinated biphenyls and polyaromatic hydrocarbons in fruits and vegetables by gas chromatography-triple quadrupole mass spectrometry with introduction of semi-quantificatio
J. Chromatogr. A, 1270 (2012), pp. 283–295
[69]
J. Wang, W. Chow, D. Leung, J. Chang
Application of ultrahigh-performance liquid chromatography and electrospray ionization quadrupole orbitrap high-resolution mass spectrometry for determination of 166 pesticides in fruits and vegetables
J. Agric. Food Chem., 60 (2012), pp. 12088–12104 http://dx.doi.org/10.1021/jf303939s
[70]
K. Banerjee, S. Mujawar, S.C. Utture, S. Dasgupta, P.G. Adsule
Optimization of gas chromatography-single quadrupole mass spectrometry conditions for multiresidue analysis of pesticides in grapes in compliance to EU-MRLs
Food Chem., 138 (2013), pp. 0–607 http://dx.doi.org/10.1016/j.foodchem.2012.10.105
[71]
K. Nagarajan, G. Khan, Z.S. Utture, S.C. Dasgupta, S. Banerjee
Ensuring selectivity and sensitivity by timed- and eltra-selective reaction monitoring during gas chromatography-tandem mass spectrometric determination of pesticides
J. Chromatogr. A, 1318 (2013), pp. 226–233
[72]
P. Sivaperumal, P. Anand, L. Riddhi
Rapid determination of pesticide residues in fruits and vegetables, using ultra-high-performance liquid chromatography/time-of-flight mass spectrometry
FOOD Chem, 168 (2015), pp. 6–365 http://dx.doi.org/10.1016/j.foodchem.2014.07.072
[73]
S. Navarro, A. Barba, G. Navarro, N. Vela, J. Olí
Multiresidue method for the rapid determination–in grape, must and wine–of fungicides frequently used on vineyards
J. Chromatogr. A, 882 (2000), pp. 221–229 www.elsevier.com
[74]
P. Caboni, G. Sarais, A. Angioni, V.L. Garau, P. Cabras
Fast and versatile multiresidue method for the analysis of botanical insecticides on fruits and vegetables by HPLC/DAD/MS
J. Agric. Food Chem., 53 (2005), pp. 8644–8649 http://dx.doi.org/10.1021/jf051345+
[75]
S. De Melo Abreu, P. Caboni, P. Cabras, V.L. Garau, A. Alves
Validation and global uncertainty of a liquid chromatographic with diode array detection method for the screening of azoxystrobin, kresoxim-methyl, trifloxystrobin, famoxadone, pyraclostrobin and fenamidone in grapes and wine
Anal. Chim. Acta, 29 (2006), pp. 537–574 http://dx.doi.org/10.1016/j.aca.2006.01.090 291–297
[76]
P. Venkateswarlu, K.R. Mohan, C.R. Kumar, K. Seshaiah
Analytical nutritional and Clinical methods monitoring of multi-class pesticide residues in fresh grape samples using liquid chromatography with electrospray tandem mass spectrometry
Food Chem., 105 (2007), pp. 1760–1766 http://dx.doi.org/10.1016/j.foodchem.2007.04.074
[77]
D.T. Likas, N.G. Tsiropoulos, G.E. Miliadis
Rapid gas chromatographic method for the determination of famoxadone, trifloxystrobin and fenhexamid residues in tomato, grape and wine samples
J. Chromatogr. A, 1150 (2007), pp. 208–214 http://dx.doi.org/10.1016/j.chroma.2006.08.041
[78]
L.M. Ravelo-Pérez, J. Hernández-Borges, M.A. Rodríguez-Delgado
Multi-walled carbon nanotubes as efficient solid-phase extraction materials of organophosphorus pesticides from apple, grape, orange and pineapple fruit juices
J. Chromatogr. A, 1211 (2008), pp. 33–42 http://dx.doi.org/10.1016/j.chroma.2008.09.084
[79]
C. Duran Guerrero, E. Natera Marin, R. Castro Mejias, R. Garcia Barroso
Traceability of phytosanitary products in the production of a sherry wine vinegar
J. Agric. Food Chem., 57 (2009), pp. 2193–2199 http://dx.doi.org/10.1021/jf803729y
[80]
S.C. Cunha, J.O. Fernandes, A. Alves, P. Oliveira
Fast low-pressure gas chromatography–mass spectrometry method for the determination of multiple pesticides in grapes, musts and wines
J. Chromatogr. A, 1216 (2009), pp. 119–126 http://dx.doi.org/10.1016/j.chroma.2008.11.015
[81]
R.M. González-Rodríguez, B. Cancho-Grande, J. Simal-Gándara
Multiresidue determination of 11 new fungicides in grapes and wines by liquidáíliquid extraction/clean-up and programmable temperature vaporization injection with analyte protectants/gas chromatography/ion trap mass spectrometry
J. Chromatogr. A, 1216 (2009), pp. 6033–6042 http://dx.doi.org/10.1016/j.chroma.2009.06.046
[82]
R. Rose, G. Lane, S. Jordan
The fate of fungicide and insecticide residues in Australian wine grape by-products following field application
Food Chem., 117 (2009), pp. 634–640
[83]
L.M. Ravelo-Pérez, J. Hernández-Borges, A.V. Herrera-Herrera, M. Ángel Rodríguez-Delgado
Pesticide extraction from table grapes and plums using ionic liquid based dispersive liquid–liquid microextraction
Anal. Bioanal. Chem., 395 (2009), pp. 2387–2395 http://dx.doi.org/10.1007/s00216-009-3133-x
[84]
I.R. Pizzutti, R.J.J. Vreuls, A. De Kok, R. Roehrs, S. Martel, C.A. Friggi, et al.
Design of a compressed air modulator to be used in comprehensive multidimensional gas chromatography and its application in the determination of pesticide residues in grapes
J. Chromatogr. A (2009), pp. 1216–3305 http://dx.doi.org/10.1016/j.chroma.2009.01.088
[85]
L. Lagunas-Allué, J. Sanz-Asensio, M.T. Martínez-Soria
Response surface optimization for determination of pesticide residues in grapes using MSPD and GC–MS: assessment of global uncertainty
Anal. Bioanal. Chem., 398 (2010), pp. 1509–1523 http://dx.doi.org/10.1007/s00216-010-4046-4
[86]
K. Banerjee, R.H. Savant, S. Dasgupta, S.H. Patil, D.P. Oulkar, P.G. Adsule
Multiresidue analysis of synthetic pyrethroid pesticides in grapes by gas chromatography with programmed temperature vaporizing-large volume injection coupled with ion trap mass spectrometry
J.AOAC Int., 93 (2010), pp. 368–379
[87]
J. Li, H.-F. Zhang, Y.-P. Shi
Monitoring multi-class pesticide residues in fresh grape by hollow fibre sorptive extraction combined with gas chromatography € mass spectrometry
Food Chem., 127 (2011), pp. 784–790 http://dx.doi.org/10.1016/j.foodchem.2010.12.148
[88]
L. Lagunas-Allue, J. Sanz-Asensio, M.T. Martinez-Soria
Validation of a microwave-assisted extraction gas chromatography detection method for the determination of fungicides in grapes
Anal Methods, 288 (2011), pp. 2881–2892 http://dx.doi.org/10.1039/c1ay05406f
[89]
C. Oellig, W. Schwack
Planar solid phase extraction-A new clean-up concept in multi-residue analysis of pesticides by liquid chromatography-mass spectrometry
J. Chromatogr. A, 1218 (2011), pp. 6540–6547 http://dx.doi.org/10.1016/j.chroma.2011.06.108
[90]
D.P. Oulkar, K. Banerjee, M.S. Ghaste, S.D. Ramteke, D.G. Naik, S.B. Patil, et al.
Multiresidue analysis of multiclass plant growth regulators in grapes by liquid chromatography/tandem mass spectrometry
J. AOAC Int. VOl., 94 (2011), pp. 968–977
[91]
M.A. Farajzadeh, D. Djozan, P. Khorram
Development of a new microextraction method based on a dynamic single drop in a narrow-bore tube: application in extraction and preconcentration of some organic pollutants in well water and grape juice samples
Talanta, 85 (2011), pp. 5–1142 http://dx.doi.org/10.1016/j.talanta.2011.05.044
[92]
S.-X. Guan, Z.-G. Yu, H.-N. Yu, C.-H. Song, Z.-q. Song, et al.
Multi-walled carbon nanotubes as matrix solid-phase dispersion extraction adsorbent for simultaneous analysis of residues of nine organophosphorus pesticides in fruit and vegetables by rapid resolution LC–MS–MS
Chromatographia, 73 (2011), pp. 33–41 http://dx.doi.org/10.1007/s10337-010-1840-2
[93]
S.N. Sinha, M. Vishnu, V. Rao, K. Vasudev, M. Odetokun
A liquid chromatography mass spectrometry-based method to measure organophosphorous insecticide, herbicide and non-organophosphorous pesticide in grape and apple samples
Food Control, 25 (2012), pp. 636–646 http://dx.doi.org/10.1016/j.foodcont.2011.11.031
[94]
P. Zhao, L. Wang, L. Zhou, F. Zhang, S. Kang, C. Pan
Multi-walled carbon nanotubes as alternative reversed-dispersive solid phase extraction materials in pesticide multi-residue analysis with QuEChERS method
J. Chromatogr. A, 1225 (2011), pp. 17–25 http://dx.doi.org/10.1016/j.chroma.2011.12.070
[95]
N. Campillo, P.V. Nas, N. Aguinaga, G. Férez, M. Hernández-Córdoba
Stir bar sorptive extraction coupled to liquid chromatography for the analysis of strobilurin fungicides in fruit samples
J. Chromatogr. A, 1217 (2010), pp. 4529–4534 http://dx.doi.org/10.1016/j.chroma.2010.05.006
[96]
E.A. Souza-Silva, V. Lopez-Avila, J. Pawliszyn
Fast and robust direct immersion solid phase microextraction coupled with gas chromatography-time-of-flight mass spectrometry method employing a matrix compatible fiber for determination of triazole fungicides in fruits
J. Chromatogr. A, 1313 (2013), pp. 139–146 http://dx.doi.org/10.1016/j.chrom.2013.07.071
[97]
M. Celeiro, M. Llompart, J.P. Lamas, M. Lores, C. Garcia-Jares, T. Dagnac
Determination of fungicides in white grape bagasse by pressurized liquid extraction and gas chromatography tandem mass spectrometry
J. Chromatogr. A, 1343 (2014), pp. 18–25 http://dx.doi.org/10.1016/j.chroma.2014.03.057
[98]
A.J. Nieto-García, Romero-Gonz, R. Alez, A. Garrido Frenich
Multi-pesticide residue analysis in nutraceuticals from grape seed extracts by gas chromatography coupled to triple quadrupole mass spectrometry
Food Control, 47 (2015), pp. 369–380 http://dx.doi.org/10.1016/j.foodcont.2014.07.041
[99]
P.A. Mills, J.H. Onley
Rapid method for chlorinated pesticide residues in non fatty foods
J. Assoc. Anal. Chem., 46 (1963), pp. 186–191
[100]
H.T. Luke, M.A. Froberg, J.E. Masumoto
Extraction and cleanup of organochlorine, organophosphate, organonitrogen, and hydrocarbon pesticides in produce for determination by gas-liquid chromatography
Food Drug Adm., 58 (1975), pp. 1020–1026
[101]
General Inspectorate for Health Protection, Analytical methods for pesticide residues in foodstuffs, 6th ed. Part 1, The Hague, The Netherlands, 1996.
[102]
M. Anastassiades, S.J. Lehotay, F.J. Schenck
Fast and easy multiresidue method employing acetonitrile extraction/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce
J.AOAC Int., 86 (2003), pp. 119–127
[103]
C. Christia, E. Bizani, C. Christophoridis, K. Fytianos
Pesticide residues in fruit samples: comparison of different QuEChERS methods using liquid chromatography–tandem mass spectrometry
Environ. Sci. Pollut. Res., 22 (2015), pp. 13167–13178
[104]
H.G.J. Mol, R.C.J. Van Dam, O.M. Steijger
Determination of polar organophosphorus pesticides in vegetables and fruits using liquid chromatography with tandem mass spectrometry: selection of extraction solvent
J. Chromatogr. A, 1015 (2003), pp. 119–127 http://dx.doi.org/10.1016/S0021-9673(03) 01209-3
[105]
L. Lagunas-Allué, J. Sanz-Asensio, M.T. Martínez-Soria
Comparison of four extraction methods for the determination of fungicide residues in grapes through gas chromatography–mass spectrometry
J. Chromatogr. A, 1270 (2012), pp. 62–71 http://dx.doi.org/10.1016/j.chroma.2012.10.069
[106]
F.J. Schenck, S.J. Lehotay, V. Vega
Comparison of solid-phase extraction sorbents for cleanup in pesticide residue analysis of fresh fruits and vegetables
J. Sep. Aci., 25 (2002), pp. 883–890
[107]
L.F.C. Melo, C.H. Collins, I.C.S.F. Jardim
New materials for solid-phase extraction and multiclass high-performance liquid chromatographic analysis of pesticides in grapes
J. Chromatogr. A, 1032 (2004), pp. 51–58
[108]
S. Grimalt, J.V. Sancho, Ó.J. Pozoa, F.E. Hernándeza
Quantification, confirmation and screening capability of UHPLC coupled to triple quadrupole and hybrid quadrupole time-of-flightmass spectrometry in pesticide residue analysis
J. Mass Spectrom., 45 (2010), pp. 421–436
[109]
M.K. Van Der Lee, G. Van Der Weg, W.A. Traag, H.G.J. Mol
Qualitative screening and quantitative determination of pesticides and contaminants in animal feed using comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry
J. Chromatogr. A, 1186 (2008), pp. 325–339 http://dx.doi.org/10.1016/j.chroma.2007.11.043
[110]
S. Grimalt, Ó.J. Pozo, J.V. Sancho, F. Hernández
Use of liquid chromatography coupled to quadrupole time-of-flight mass spectrometry to investigate pesticide residues in fruits
Anal. Chem., 79 (2007), pp. 2833–2843 http://dx.doi.org/10.1021/ac061233x
[111]
C.D. Stalikas, C.N. Konidari
Analytical methods to determine phosphonic and amino acid group-containing pesticides
J. Chromatogr. A, 907 (2001), pp. 1–19 www.elsevier.com
[112]
J. Bernal, A.M. Ares, J. Pól, S.K. Wiedmer
Hydrophilic interaction liquid chromatography in food analysis
J. Chromatogr. A, 1218 (2011), pp. 7438–7452 http://dx.doi.org/10.1016/j.chroma.2011.05.004
[113]
D. Anastassiades, M. Kolberg, D.I. Mack, D. Wildgrube, C. Sigalov, I. Dork, Quick Method for the Analysis of Residues of numerous Highly Polar Pesticides in Foods of Plant Origin involving Simultaneous Extraction with Methanol and LC–MS/MS Determination (QuPPe-Method), EURL-SRM. (2013) 434. http://www.crl-pesticides.eu/librry/docs/srm/meth_QuPPe.pdf.
[114]
H.G.J. Mol, R.C.J. Van Dam
Rapid detection of pesticides not amenable to multi-residue methods by flow injection–tandem mass spectrometry
Anal. Bioanal. Chem., 406 (2014), pp. 66–79 http://dx.doi.org/10.1007/s00216-014-7644-8
[115]
V. Hanot, L. Joly, A. Bonnechère, J. Van Loco
Rapid determination of ethephon in grapes by hydrophilic interaction chromatography tandem mass spectrometry
Food Anal. Methods, 8 (2015), pp. 524–530 http://dx.doi.org/10.1007/s12161-014-9921-8
[116]
W. Crnogorac, G. Schwack
Residue analysis of dithiocarbamate fungicides
Trends Anal. Chem., 28 (2009), pp. 40–50 http://dx.doi.org/10.1016/j.trac.2008.10.08
[117]
T. Portolés, J.G.J. Mol, J.V. Sancho, F.J. López, F. Hernández
Validation of a qualitative screening method for pesticides in fruits and vegetables by gas chromatography quadrupole-time of flight mass spectrometry with atmospheric pressure chemical ionization
Anal. Chim. Acta, 838 (2014), pp. 76–85 http://dx.doi.org/10.1016/j.aca.2014.06.006
[118]
C. Ferrer, M.J. Martinez-Bueno, A. Lozano, A.R. Fernandez-Alba
Pesticide residue analysis of fruit juices by LC–MS/MS direct injection. One year pilot survey
Talanta, 83 (2011), pp. 1552–1561 http://dx.doi.org/10.1016/j.talanta.2010.11.061
[119]
S.C. Nanita
Quantitative mass spectrometry independence from matrix effects and detector saturation achieved by flow injection analysis with real-time infinite dilution
Anal. Chem., 85 (2013), pp. 11866–11875 http://dx.doi.org/10.1021/ac402567w
[120]
C. Oellig, W. Schwack
Planar solid phase extraction clean-up and microliter-flow injection analysis-time-of-flight mass spectrometry for multi-residue screening of pesticides in food
J. Chromatogr. A, 1351 (2014), pp. 1–11 http://dx.doi.org/10.1016/j.chroma.2014.05.032
[121]
S.E. Edison, L.A. Lin, B.M. Gamble, J. Wong, K. Zhang
Surface swabbing technique for the rapid screening for pesticides using ambient pressure desorption ionization with high-resolution mass spectrometry
Rapid Commun. Mass Spectrom., 25 (2011), pp. 127–139 http://dx.doi.org/10.1002/rcm.4831
[122]
B. Wang, X. Ding, Z. Zhao, Y. Duan
Method Development for Directly Screening Pesticide Residues in Foodstuffs Using Ambient Microfabricated Glow Discharge Plasma (mfgdp) Desorption/ionization Mass Spectrometry
Elsevier B.V (2014) http://dx.doi.org/10.1016/j.ijms.2014.05.018
[123]
A. Albert, A. Kramer, S. Scheeren, C. Engelhard
Rapid and quantitative analysis of pesticides in QuEChERS pretreatmetn and low-temperature plasma desorption/ionization orbitrap mass spectrometry
Anal. Methods, 6 (2014), pp. 5463–5471 http://dx.doi.org/10.1039/c4ay00103r
